VolumeView VLV6R520
GUDID 00690103187007
VOLUMEVIEW SET,5F,20CM,60",1P
Edwards Lifesciences LLC
Peripheral vascular catheter| Primary Device ID | 00690103187007 |
| NIH Device Record Key | 6c487ca4-76b7-4ff0-8620-665c7ce09364 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | VolumeView |
| Version Model Number | VLV6R520 |
| Catalog Number | VLV6R520 |
| Company DUNS | 134139174 |
| Company Name | Edwards Lifesciences LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)822-9637 |
| tech_support@edwards.com | |
| Phone | +1(800)822-9637 |
| tech_support@edwards.com |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool, dry place. |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool, dry place. |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00690103187007 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K100739
FDA Product Code
| KRB | PROBE, THERMODILUTION |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-09-03 |
On-Brand Devices [VolumeView]
| 00690103190427 | CV THERMISTOR KIT 5 PACK |
| 00690103187076 | VOLUMEVIEW SET,4F,16CM,60",5P |
| 00690103187069 | VOLUMEVIEW SET,4F,16CM,84",5P |
| 00690103187052 | VOLUMEVIEW SET,4F,16CM,84",1P |
| 00690103187045 | VOLUMEVIEW SET,4F,16CM,60",1P |
| 00690103187038 | VOLUMEVIEW SET,5F,20CM,60",5P |
| 00690103187021 | VOLUMEVIEW COMBO KIT |
| 00690103187014 | VOLUMEVIEW SET,5F,20CM,84",1P |
| 00690103187007 | VOLUMEVIEW SET,5F,20CM,60",1P |
| 00690103185485 | FEMORAL CATHETER KIT (4F, 16CM) |
| 00690103183924 | FEMORAL CATHETER KIT |
| 00690103183917 | 1-FEMORAL CATHETER KIT (5F, 20 CM), SINGLE PACK |
| 00690103183900 | CV THERMISTOR KIT 5 PACK |
| 07460691950481 | VOLUMEVIEW SENSOR,84",5P,RED C |
Trademark Results [VolumeView]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
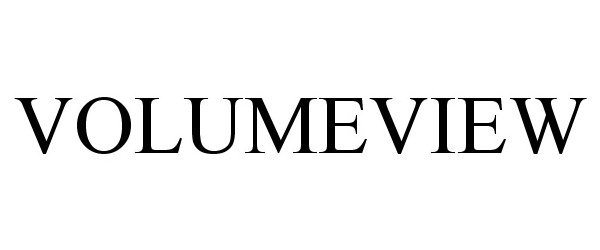 VOLUMEVIEW 86164407 4758354 Live/Registered |
Edwards Lifesciences Corporation 2014-01-13 |
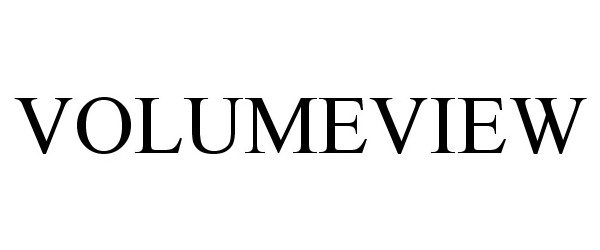 VOLUMEVIEW 85294487 not registered Dead/Abandoned |
Edwards Lifesciences Corporation 2011-04-13 |
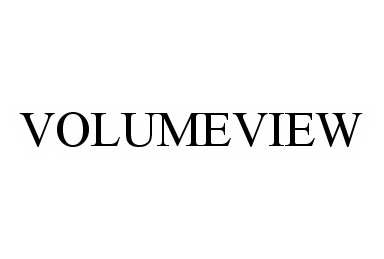 VOLUMEVIEW 78497857 not registered Dead/Abandoned |
Chroma Medical Systems, Inc. 2004-10-11 |
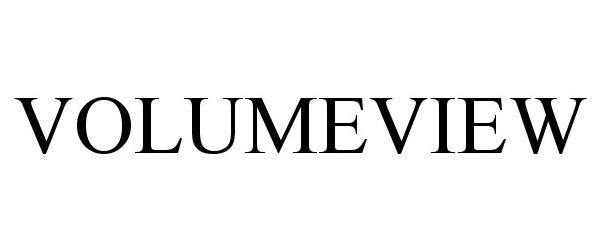 VOLUMEVIEW 77693770 not registered Dead/Abandoned |
Edwards :Lifesciences Corporation 2009-03-18 |
 VOLUMEVIEW 73830037 1626779 Dead/Cancelled |
INNOVATIVE IMAGING SYSTEMS, INC. 1989-10-10 |
 VOLUMEVIEW 73663934 1474597 Dead/Cancelled |
INNOVATIVE IMAGING SYSTEMS, INC. 1987-06-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.