AquaDerm
GUDID 00714196322227
DERMARITE INDUSTRIES LLC
Wound hydrogel dressing, non-antimicrobial| Primary Device ID | 00714196322227 |
| NIH Device Record Key | d9d5c24c-56f4-481c-986a-75d4bf8a154b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | AquaDerm |
| Version Model Number | 00322E |
| Company DUNS | 883925562 |
| Company Name | DERMARITE INDUSTRIES LLC |
| Device Count | 10 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00714196322227 [Primary] |
| GS1 | 01714196322226 [Unit of Use] |
FDA Product Code
| NAE | Dressing, Wound, Hydrogel Without Drug And/Or Biologic |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-09-09 |
| Device Publish Date | 2020-09-01 |
On-Brand Devices [AquaDerm]
| 00714196324443 | 00324E |
| 00714196322227 | 00322E |
| 00714196390042 | 00324E |
| 00714196390028 | 00322E |
Trademark Results [AquaDerm]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AQUADERM 88320615 not registered Live/Pending |
LAPKO Inc. 2019-02-28 |
 AQUADERM 78777758 3180503 Dead/Cancelled |
Lanxess Deutschland GmbH 2005-12-21 |
 AQUADERM 74663213 1986866 Dead/Cancelled |
Bayer Aktiengesellschaft 1995-04-19 |
 AQUADERM 73757881 1578499 Dead/Cancelled |
BAKER CUMMINS LABORATORIES, INC. 1988-10-17 |
 AQUADERM 73595621 not registered Dead/Abandoned |
LABORATOIRES ED. FROMONT 1986-04-28 |
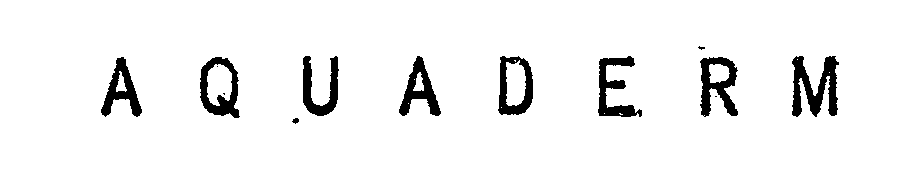 AQUADERM 72183084 0779351 Dead/Expired |
MENLEY & JAMES LABORATORIES, LTD. 1963-12-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.