TYRX™
GUDID 00763000101626
ENVELOPE NMRM6122 NEURO ABSORB MED US
MEDTRONIC, INC.
Implantable pulse generator mesh bag, bioabsorbable| Primary Device ID | 00763000101626 |
| NIH Device Record Key | 216c0ebe-36b9-486c-8b98-2b0b23a5586e |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | TYRX™ |
| Version Model Number | NMRM6122 |
| Company DUNS | 006261481 |
| Company Name | MEDTRONIC, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Safe |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | true |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com |
Device Dimensions
| Width | 2.7 Inch |
| Width | 2.7 Inch |
| Width | 2.7 Inch |
| Width | 2.7 Inch |
| Length | 2.5 Inch |
| Width | 2.7 Inch |
| Length | 2.5 Inch |
| Width | 2.7 Inch |
| Length | 2.5 Inch |
| Width | 2.7 Inch |
| Length | 2.5 Inch |
| Width | 2.7 Inch |
| Length | 2.5 Inch |
| Width | 2.7 Inch |
| Length | 2.5 Inch |
| Width | 2.7 Inch |
| Length | 2.5 Inch |
| Width | 2.7 Inch |
| Length | 2.5 Inch |
| Width | 2.7 Inch |
| Length | 2.5 Inch |
| Width | 2.7 Inch |
| Length | 2.5 Inch |
| Width | 2.7 Inch |
| Length | 2.5 Inch |
| Width | 2.7 Inch |
Operating and Storage Conditions
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 36 Degrees Fahrenheit and 77 Degrees Fahrenheit |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00763000101626 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K180122
FDA Product Code
| FTL | Mesh, surgical, polymeric |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2018-08-29 |
| Device Publish Date | 2018-07-29 |
On-Brand Devices [TYRX™]
| 00643169983540 | ENVELOPE NMRM6133 ABSORB LRG US MKT |
| 00643169983533 | ENVELOPE NMRM6122 ABSORB MED US MKT |
| 00643169935907 | ENVELOPE CMRM6133 ABSORB LRG US |
| 00643169935891 | ENVELOPE CMRM6122 ABSORB MED US |
| 00763000101633 | ENVELOPE NMRM6133 NEURO ABSORB LRG US |
| 00763000101626 | ENVELOPE NMRM6122 NEURO ABSORB MED US |
| 00763000101619 | ENVELOPE CMRM6133 ABSORB LRG US |
| 00763000101602 | ENVELOPE CMRM6122 ABSORB MED US |
| 00763000307882 | ENVELOPE CMRM6133 ABSORB LRG MR |
| 00763000307875 | ENVELOPE CMRM6122 ABSORB MED MR |
| 00763000307905 | ENVELOPE NMRM6133 ABSORB LRG MR |
| 00763000307899 | ENVELOPE NMRM6122 ABSORB MED MR |
Trademark Results [TYRX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TYRX 86213023 4736448 Live/Registered |
MEDTRONIC, INC. 2014-03-06 |
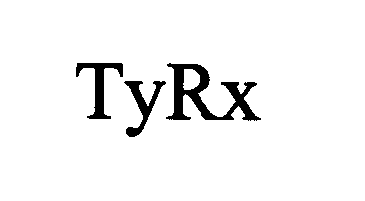 TYRX 76571469 3022471 Live/Registered |
MEDTRONIC, INC. 2004-01-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.