Duet®
GUDID 00763000428723
BAG 46911 DRAINAGE DEHP-FREE 1PK
MEDTRONIC PS MEDICAL, INC.
Cerebrospinal fluid external drainage kit| Primary Device ID | 00763000428723 |
| NIH Device Record Key | c10aeab8-59b5-4d43-b538-617f2d4de41d |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Duet® |
| Version Model Number | 46911 |
| Company DUNS | 089055867 |
| Company Name | MEDTRONIC PS MEDICAL, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00763000428723 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- deNovo: DEN120017
FDA Product Code
| JXG | Shunt, central nervous system and components |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-03-15 |
| Device Publish Date | 2021-03-07 |
On-Brand Devices [Duet®]
| 00613994445407 | EDMS 46917 DUET INTERLINK WITH LCATH |
| 00613994445391 | EDMS 46916 DUET BLUECORE WITH VCATH |
| 00613994445384 | EDMS 46915 DUET INTERLINK WITH VCATH |
| 00613994445377 | EDMS 46914 DUET BLUECORE SITES |
| 00613994445360 | EDMS 46913 DUET INTERLINK SITES |
| 20613994430537 | BAG 46912 DRAINAGE DEHP-FREE 10PK |
| 00613994430526 | BAG 46911 DRAINAGE DEHP-FREE 1PK |
| 00763000396008 | EDMS DUET 46915 INTERLINK W VCATH |
| 00763000431235 | BAG 46911 DRAINAGE DEHP-FREE 1PK |
| 00763000406028 | EDMS 46917 DUET INTERLINK WITH LCATH |
| 00763000406011 | EDMS 46916 DUET BLUECORE WITH VCATH |
| 00763000406004 | EDMS 46913 DUET INTERLINK SITES |
| 00763000395971 | EDMS DUET 46914 SMARTSITE SITES |
| 20763000431277 | BAG 46912 DRAINAGE DEHP-FREE 10PK |
| 00763000428785 | EDMS 46917 DUET INTERLINK WITH LCATH |
| 00763000428778 | EDMS 46916 DUET BLUECORE WITH VCATH |
| 00763000428761 | EDMS DUET 46915 INTERLINK W VCATH |
| 00763000428754 | EDMS DUET 46914 SMARTSITE SITES |
| 00763000428747 | EDMS 46913 DUET INTERLINK SITES |
| 00763000428730 | BAG 46912 DRAINAGE DEHP-FREE 10PK |
| 00763000428723 | BAG 46911 DRAINAGE DEHP-FREE 1PK |
| 00763000624804 | EDMS 46917 DUET INTERLINK WITH LCATH |
| 00763000624798 | EDMS 46916 DUET BLUECORE WITH VCATH |
| 00763000624781 | EDMS DUET 46915 INTERLINK W VCATH |
| 00763000624774 | EDMS DUET 46914 SMARTSITE SITES |
| 00763000624767 | EDMS 46913 DUET INTERLINK SITES |
| 00763000624750 | BAG 46912 DRAINAGE DEHP-FREE 10PK |
| 00763000624743 | BAG 46911 DRAINAGE DEHP-FREE 1PK |
Trademark Results [Duet]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DUET 98777624 not registered Live/Pending |
Enurgen Inc. 2024-09-30 |
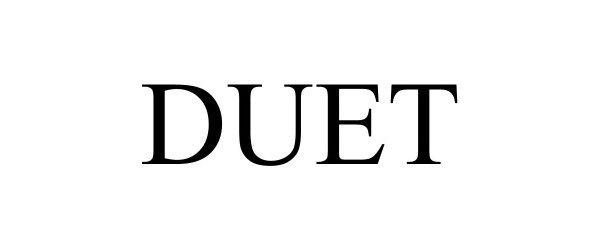 DUET 98333439 not registered Live/Pending |
Medical Depot, Inc. 2023-12-28 |
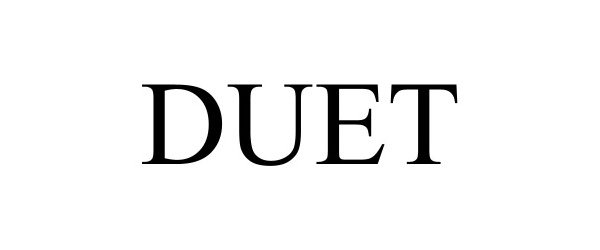 DUET 98225435 not registered Live/Pending |
SSCOR, INC. 2023-10-16 |
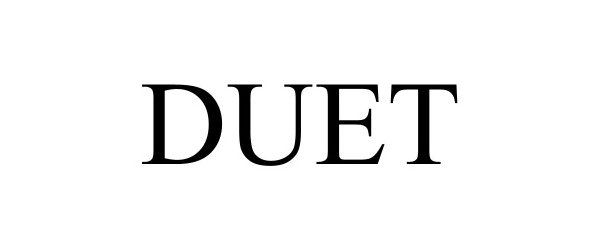 DUET 98125942 not registered Live/Pending |
Cerillo, Inc 2023-08-10 |
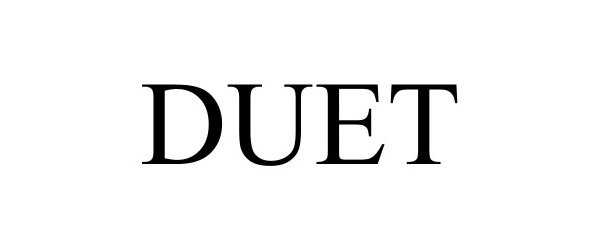 DUET 98101325 not registered Live/Pending |
Plan With Duet, Inc. 2023-07-25 |
 DUET 97978824 not registered Live/Pending |
Wink Limited 2023-04-13 |
 DUET 97886644 not registered Live/Pending |
Wink Limited 2023-04-13 |
 DUET 97865887 not registered Live/Pending |
KUPA, INC. 2023-03-30 |
 DUET 97616275 not registered Live/Pending |
STYLEADDICT LLC 2022-10-02 |
 DUET 97587718 not registered Live/Pending |
Peak Outcomes, LLC 2022-09-12 |
 DUET 97526334 not registered Live/Pending |
Wells Fargo & Company 2022-07-29 |
 DUET 97340530 not registered Live/Pending |
Duet Preservation Audiology, Public Benefit Corporation 2022-03-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.