FLEXTEND®
GUDID 00802526397745
Steroid-Eluting Pace/Sense Endocardial Lead
BOSTON SCIENTIFIC CORPORATION
Endocardial pacing lead| Primary Device ID | 00802526397745 |
| NIH Device Record Key | 00389ba6-50ac-45dd-a712-c9b2d4478b47 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | FLEXTEND® |
| Version Model Number | 4088 |
| Company DUNS | 106295384 |
| Company Name | BOSTON SCIENTIFIC CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | true |
| Single Use | true |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00802526397745 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Approval: P960006
FDA Product Code
| DTB | permanent pacemaker Electrode |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2014-09-24 |
On-Brand Devices [FLEXTEND®]
| 00802526397745 | Steroid-Eluting Pace/Sense Endocardial Lead |
| 00802526397738 | Steroid-Eluting Pace/Sense Endocardial Lead |
| 00802526397721 | Steroid-Eluting Pace/Sense Endocardial Lead |
Trademark Results [FLEXTEND]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FLEXTEND 86193086 not registered Dead/Abandoned |
Shackelton Inc. 2014-02-13 |
 FLEXTEND 79212053 5494520 Live/Registered |
AGSPEC AUSTRALIA PTY LTD 2017-05-23 |
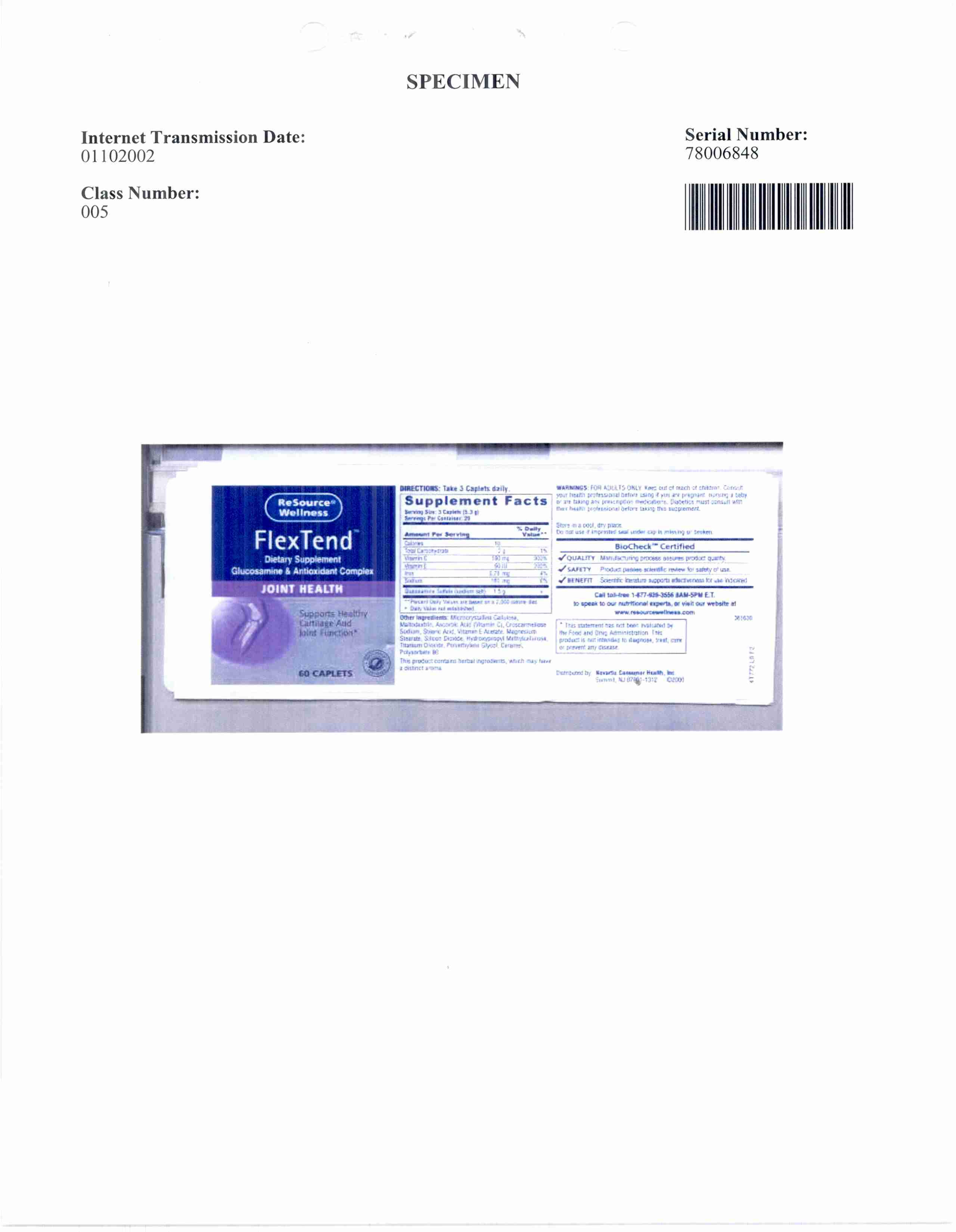 FLEXTEND 78006848 2583799 Dead/Cancelled |
SOCIETE DES PRODUITS NESTLE, S.A. 2000-05-04 |
 FLEXTEND 77171366 3499735 Dead/Cancelled |
Ariens Company 2007-05-02 |
 FLEXTEND 76570898 2988585 Dead/Cancelled |
Lancome Parfums et Beaute & Cie 2004-01-16 |
 FLEXTEND 75888356 2473115 Live/Registered |
Factory Automation Systems, Inc. 2000-01-06 |
 FLEXTEND 75604218 2311560 Live/Registered |
Hollister Incorporated 1998-12-14 |
 FLEXTEND 75540497 2390346 Live/Registered |
Cardiac Pacemakers, Inc. 1998-08-21 |
 FLEXTEND 75391445 2205752 Live/Registered |
ANLIKER, JEFF P., MR. 1997-11-17 |
 FLEXTEND 74715735 not registered Dead/Abandoned |
WORLDWIDE MARKETING PARTNERS 1995-08-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.