INGEVITY™ MRI
GUDID 00802526523489
Endocardial Steroid-eluting MR Conditional Stylet delivered Pace/Sense Lead
BOSTON SCIENTIFIC CORPORATION
Endocardial pacing lead| Primary Device ID | 00802526523489 |
| NIH Device Record Key | a28e80f0-3c4e-475b-96e2-ee13c3c3496e |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | INGEVITY™ MRI |
| Version Model Number | 7742 |
| Company DUNS | 106295384 |
| Company Name | BOSTON SCIENTIFIC CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Conditional |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | true |
| Single Use | true |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00802526523489 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Approval: P150012
FDA Product Code
| NVN | Drug eluting permanent right ventricular (RV) or right atrial (RA) pacemaker electrodes |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 5 |
| Public Version Date | 2019-06-07 |
| Device Publish Date | 2016-05-10 |
On-Brand Devices [INGEVITY™ MRI]
| 00802526523489 | Endocardial Steroid-eluting MR Conditional Stylet delivered Pace/Sense Lead |
| 00802526523458 | Endocardial Steroid-eluting MR Conditional Stylet delivered Pace/Sense Lead |
| 00802526523427 | Endocardial Steroid-eluting MR Conditional Stylet delivered Pace/Sense Lead |
| 00802526523397 | Endocardial Steroid-eluting MR Conditional Stylet delivered Pace/Sense Lead |
| 00802526523366 | Endocardial Steroid-eluting MR Conditional Stylet delivered Pace/Sense Lead |
| 00802526523335 | Endocardial Steroid-eluting MR Conditional Stylet delivered Pace/Sense Lead |
| 00802526523304 | Endocardial Steroid-eluting MR Conditional Stylet delivered Pace/Sense Lead |
Trademark Results [INGEVITY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
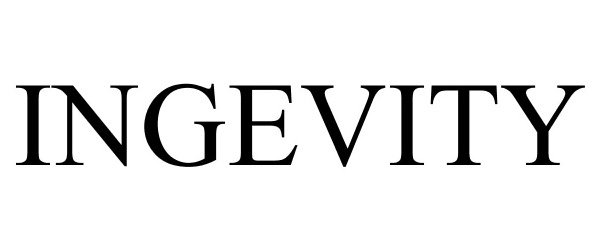 INGEVITY 86827644 5088206 Live/Registered |
Ingevity South Carolina, LLC 2015-11-20 |
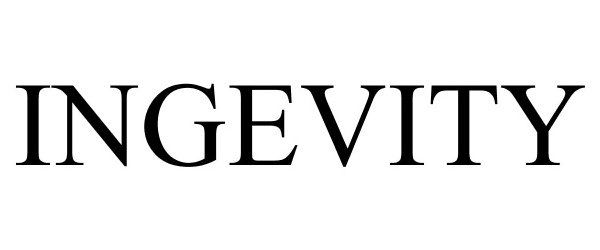 INGEVITY 86821741 5092710 Live/Registered |
Cardiac Pacemakers, Inc. 2015-11-16 |
 INGEVITY 86748345 5087931 Live/Registered |
INGEVITY SOUTH CAROLINA, LLC 2015-09-04 |
 INGEVITY 86748336 5087930 Live/Registered |
INGEVITY SOUTH CAROLINA, LLC 2015-09-04 |
 INGEVITY 86748323 5087929 Live/Registered |
INGEVITY SOUTH CAROLINA, LLC 2015-09-04 |
 INGEVITY 86748312 5087928 Live/Registered |
INGEVITY SOUTH CAROLINA, LLC 2015-09-04 |
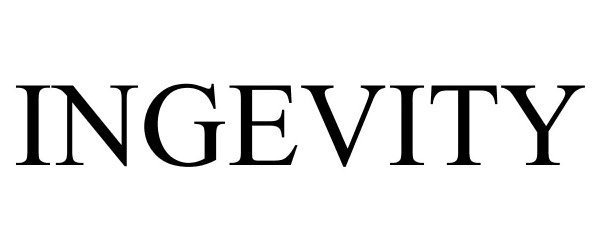 INGEVITY 85677818 not registered Dead/Abandoned |
Cardiac Pacemakers, Inc. 2012-07-16 |
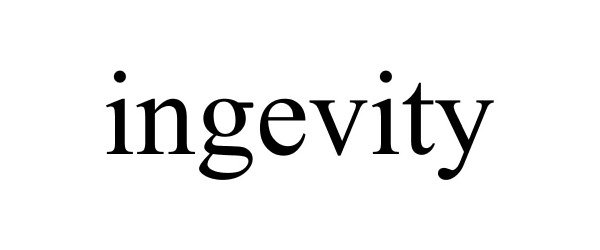 INGEVITY 77162011 3411432 Dead/Cancelled |
Burns, Fraser C 2007-04-20 |
 INGEVITY 76694769 not registered Dead/Abandoned |
Cardiac Pacemakers, Inc. 2008-12-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.