DYNAGEN™ CRT-D
GUDID 00802526534638
Cardiac Resynchronization Therapy Defibrillator
BOSTON SCIENTIFIC CORPORATION
Cardiac resynchronization therapy implantable defibrillator| Primary Device ID | 00802526534638 |
| NIH Device Record Key | fe3e7476-982b-4bb4-a7a0-1ab510074beb |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | DYNAGEN™ CRT-D |
| Version Model Number | G151 |
| Company DUNS | 106295384 |
| Company Name | BOSTON SCIENTIFIC CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Conditional |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00802526534638 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Approval: P010012
FDA Product Code
| NIK | Defibrillator, automatic implantable cardioverter, with cardiac resynchronization (CRT-D) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 5 |
| Public Version Date | 2019-12-06 |
| Device Publish Date | 2014-11-06 |
On-Brand Devices [DYNAGEN™ CRT-D]
| 00802526534652 | Cardiac Resynchronization Therapy Defibrillator |
| 00802526534638 | Cardiac Resynchronization Therapy Defibrillator |
| 00802526534614 | Cardiac Resynchronization Therapy Defibrillator |
Trademark Results [DYNAGEN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
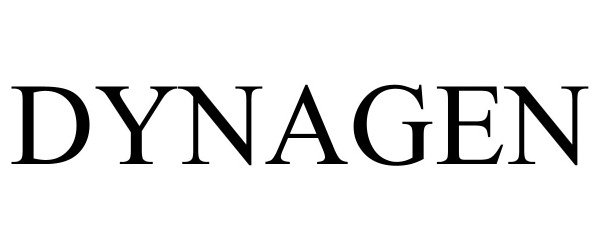 DYNAGEN 98255507 not registered Live/Pending |
Illinois Tool Works Inc. 2023-11-05 |
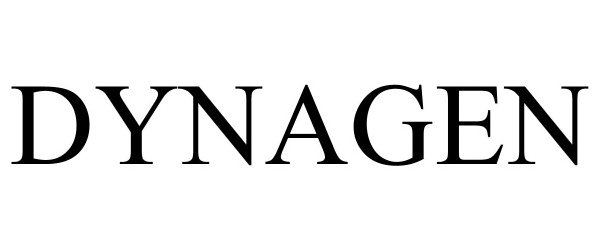 DYNAGEN 97485129 not registered Live/Pending |
Cattron North America, Inc. 2022-07-01 |
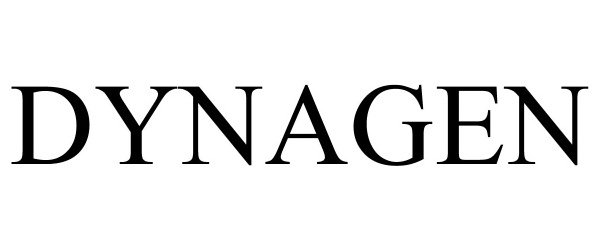 DYNAGEN 97220562 not registered Live/Pending |
Cattron North America, Inc. 2022-01-14 |
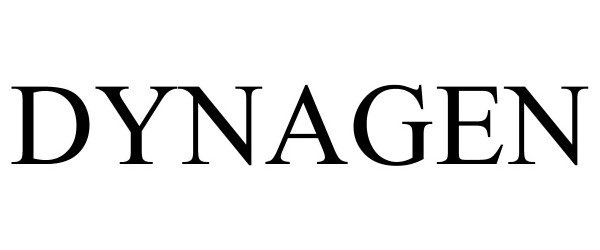 DYNAGEN 88918751 not registered Live/Pending |
Illinois Tool Works, Inc. 2020-05-15 |
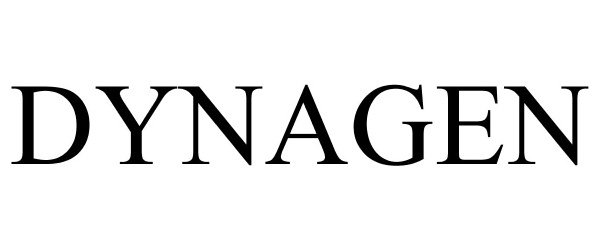 DYNAGEN 86316764 4715161 Live/Registered |
DynaGen Technologies, Inc. 2014-06-22 |
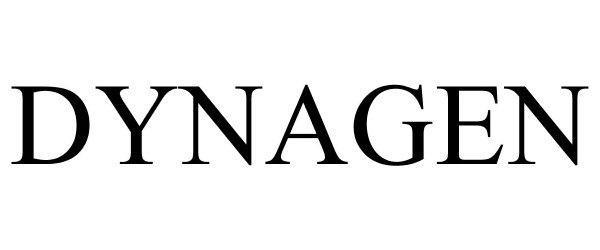 DYNAGEN 85608132 4863471 Live/Registered |
Boston Scientific Scimed, Inc. 2012-04-25 |
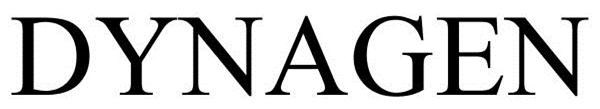 DYNAGEN 85105300 4246449 Live/Registered |
ISP INVESTMENTS LLC 2010-08-11 |
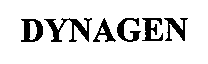 DYNAGEN 76658839 3207260 Dead/Cancelled |
ILLINOIS TOOL WORKS INC. 2006-04-20 |
 DYNAGEN 76171836 not registered Dead/Abandoned |
ANALYTICAL SENSORS, INC. 2000-11-28 |
 DYNAGEN 75851860 2410542 Live/Registered |
Intergraph Corporation 1999-11-17 |
 DYNAGEN 75128854 not registered Dead/Abandoned |
Variable Fuel Technologies Group, Inc. 1996-07-02 |
 DYNAGEN 73594856 1425569 Dead/Cancelled |
SOFTWARE DYNAMICS CORPORATION 1986-04-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.