RELIANCE 4-FRONT™
GUDID 00802526592829
Steroid-eluting Bipolar Pace/Sense and Defibrillation Lead
BOSTON SCIENTIFIC CORPORATION
Endocardial defibrillation lead| Primary Device ID | 00802526592829 |
| NIH Device Record Key | 8e66eb97-ed98-4bf3-8e29-d3f839fb4292 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | RELIANCE 4-FRONT™ |
| Version Model Number | 0672 |
| Company DUNS | 106295384 |
| Company Name | BOSTON SCIENTIFIC CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Conditional |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | true |
| Single Use | true |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00802526592829 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Approval: P910073
FDA Product Code
| NVY | Permanent defibrillator electrodes |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2019-10-09 |
| Device Publish Date | 2018-08-25 |
On-Brand Devices [RELIANCE 4-FRONT™]
| 00802526592874 | Steroid-eluting Bipolar Pace/Sense and Defibrillation Lead |
| 00802526592867 | Steroid-eluting Bipolar Pace/Sense and Defibrillation Lead |
| 00802526592850 | Steroid-eluting Bipolar Pace/Sense and Defibrillation Lead |
| 00802526592843 | Steroid-eluting Bipolar Pace/Sense and Defibrillation Lead |
| 00802526592836 | Steroid-eluting Bipolar Pace/Sense and Defibrillation Lead |
| 00802526592829 | Steroid-eluting Bipolar Pace/Sense and Defibrillation Lead |
| 00802526592812 | Steroid-eluting Bipolar Pace/Sense and Defibrillation Lead |
| 00802526592805 | Steroid-eluting Bipolar Pace/Sense and Defibrillation Lead |
| 00802526497001 | Steroid-eluting Bipolar Pace/Sense and Defibrillation Lead |
| 00802526496981 | Steroid-eluting Bipolar Pace/Sense and Defibrillation Lead |
| 00802526496967 | Steroid-eluting Bipolar Pace/Sense and Defibrillation Lead |
| 00802526496868 | Steroid-eluting Bipolar Pace/Sense and Defibrillation Lead |
Trademark Results [RELIANCE 4-FRONT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RELIANCE 4-FRONT 87599526 5885914 Live/Registered |
Cardiac Pacemakers, Inc. 2017-09-07 |
 RELIANCE 4-FRONT 86233013 not registered Dead/Abandoned |
Cardiac Pacemakers, Inc. 2014-03-26 |
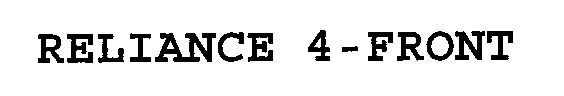 RELIANCE 4-FRONT 76704342 not registered Dead/Abandoned |
Cardiac Pacemakers, Inc. 2010-09-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.