LUX-Dx™ 667256-110
GUDID 00802526625404
For use with Insertable Cardiac Monitor system
BOSTON SCIENTIFIC CORPORATION
Implantable cardiac device management application software| Primary Device ID | 00802526625404 |
| NIH Device Record Key | 55aadc0d-72bf-4d2b-b930-b8ed038a005d |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | LUX-Dx™ |
| Version Model Number | 667256-110 |
| Catalog Number | 667256-110 |
| Company DUNS | 106295384 |
| Company Name | BOSTON SCIENTIFIC CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Unsafe |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00802526625404 [Primary] |
FDA Product Code
| MXD | Recorder, event, implantable cardiac, (with arrhythmia detection) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-10-08 |
| Device Publish Date | 2024-09-30 |
Devices Manufactured by BOSTON SCIENTIFIC CORPORATION
| 08714729058632 - Contour™ | 2026-02-17 Ureteral Stent Set |
| 08714729058953 - Contour™ | 2026-02-17 Ureteral Stent Set |
| 08714729297253 - Percuflex™ Plus | 2026-02-17 Ureteral Stent Set |
| 08714729297352 - Percuflex™ Plus | 2026-02-17 Ureteral Stent Set |
| 08714729297611 - Percuflex™ Plus | 2026-02-17 Ureteral Stent Set |
| 08714729339830 - Percuflex™ | 2026-02-17 Ureteral Stent Set |
| 08714729424123 - Contour™ | 2026-02-17 Ureteral Stent Set |
| 08714729424222 - Contour™ | 2026-02-17 Ureteral Stent Set |
Trademark Results [LUX-Dx]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
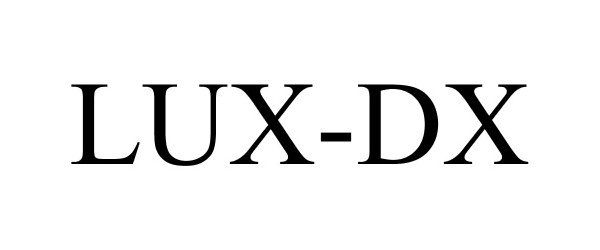 LUX-DX 87569443 not registered Dead/Abandoned |
Cardiac Pacemakers, Inc. 2017-08-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.