CLARISSE
GUDID 00808829125672
Human chorionic gonadotropin (HCG) test system intended for the early detection of pregnancy - (1) Identification. A human chorionic gonadotropin (HCG) test system is a device intended for the early detection of pregnancy is intended to measure HCG, a placental hormone, in plasma or urine.
DELTA BRANDS INC.
Total human chorionic gonadotropin IVD, kit, rapid ICT, clinical| Primary Device ID | 00808829125672 |
| NIH Device Record Key | 0eb48c7b-18c9-4e70-9691-25782a9f6190 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | CLARISSE |
| Version Model Number | Test midstream I |
| Company DUNS | 102672008 |
| Company Name | DELTA BRANDS INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | true |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00808829125672 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K183097
FDA Product Code
| LCX | Kit, Test, Pregnancy, Hcg, Over The Counter |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-03-15 |
| Device Publish Date | 2024-03-07 |
On-Brand Devices [CLARISSE]
| 00808829111903 | Cassette |
| 00808829120615 | Over-the-counter, One Step human Chorionic Gonadotropin hCG. Colloidal Gold Urine Pregnancy. fo |
| 00808829125672 | Human chorionic gonadotropin (HCG) test system intended for the early detection of pregnancy - ( |
Trademark Results [CLARISSE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CLARISSE 79113588 4452844 Live/Registered |
ISOTROPIX 2012-04-27 |
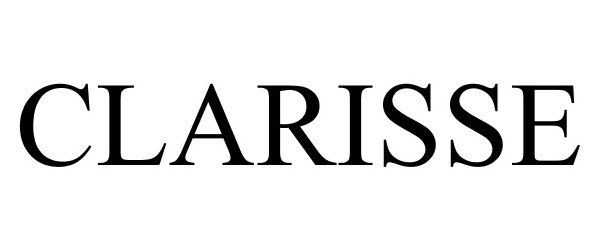 CLARISSE 78979102 3346348 Live/Registered |
CLARISSE, INC. 2005-10-17 |
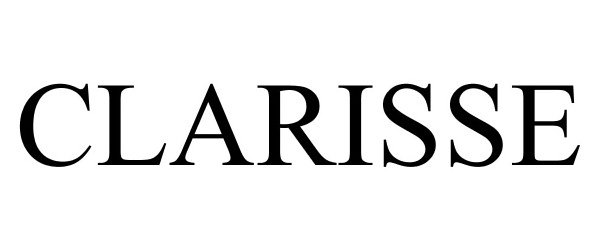 CLARISSE 78857599 not registered Dead/Abandoned |
Delta Brands Inc. 2006-04-10 |
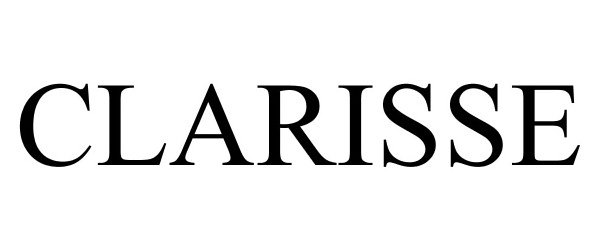 CLARISSE 78734351 not registered Dead/Abandoned |
Promgirl, Inc. 2005-10-17 |
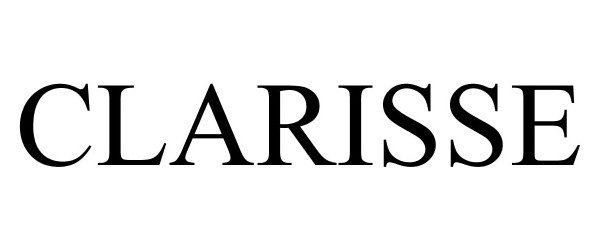 CLARISSE 77983605 4522171 Live/Registered |
Delta Brands Inc. 2010-02-04 |
 CLARISSE 77928329 not registered Dead/Abandoned |
Delta Brands Inc. 2010-02-04 |
 CLARISSE 77755490 3773448 Live/Registered |
CLARISSE, INC. 2009-06-09 |
 CLARISSE 77707749 not registered Dead/Abandoned |
Promgirl, Inc. 2009-04-06 |
 CLARISSE 77707746 3808151 Live/Registered |
CLARISSE, INC. 2009-04-06 |
 CLARISSE 77707742 4122137 Dead/Cancelled |
CLARISSE, INC. 2009-04-06 |
 CLARISSE 74065040 not registered Dead/Abandoned |
Saramar Corporation 1990-06-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.