CoLink Vallux
GUDID 00810021866291
CoLink Vallux Plate
IN2BONES USA, LLC
Internal orthopaedic fixation system, plate/screw, non-bioabsorbable, sterile| Primary Device ID | 00810021866291 |
| NIH Device Record Key | 0c9179e1-a662-4aa1-91f9-060923b50a97 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | CoLink Vallux |
| Version Model Number | P90 ST031 |
| Company DUNS | 079700711 |
| Company Name | IN2BONES USA, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00810021866291 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K212487
FDA Product Code
| HRS | Plate, Fixation, Bone |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-01-31 |
| Device Publish Date | 2022-01-21 |
Devices Manufactured by IN2BONES USA, LLC
| 30810175501001 - IBS-B MIS | 2026-02-02 IBS-B MIS Compression Screw, Ø3.3x16mm |
| 30810175501018 - IBS-B MIS | 2026-02-02 IBS-B MIS Compression Screw, Ø3.3x18mm |
| 30810175501025 - IBS-B MIS | 2026-02-02 IBS-B MIS Compression Screw, Ø3.3x20mm |
| 30810175501032 - IBS-B MIS | 2026-02-02 IBS-B MIS Compression Screw, Ø3.3x22mm |
| 30810175501049 - IBS-B MIS | 2026-02-02 IBS-B MIS Compression Screw, Ø3.3x24mm |
| 30810175501056 - IBS-B MIS | 2026-02-02 IBS-B MIS Compression Screw, Ø3.3x26mm |
| 30810175501063 - IBS-B MIS | 2026-02-02 IBS-B MIS Compression Screw, Ø3.3x28mm |
| 30810175501070 - IBS-B MIS | 2026-02-02 IBS-B MIS Compression Screw, Ø3.3x30mm |
Trademark Results [CoLink Vallux]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
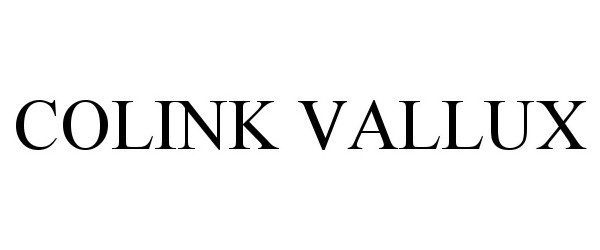 COLINK VALLUX 90494015 not registered Live/Pending |
In2Bones USA, LLC 2021-01-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.