Lucid Hearing® 10076-BHA
GUDID 00810068832730
BHA-Engage-OTC-I-BG
HEARING LAB TECHNOLOGY, LLC
Air-conduction hearing aid, receiver-in-canal| Primary Device ID | 00810068832730 |
| NIH Device Record Key | 68e6bee3-2f9e-42d9-a51f-d838af0549aa |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Lucid Hearing® |
| Version Model Number | 759* |
| Catalog Number | 10076-BHA |
| Company DUNS | 827693503 |
| Company Name | HEARING LAB TECHNOLOGY, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | MR Unsafe |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com | |
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com | |
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com | |
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com | |
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com | |
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com | |
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com | |
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com | |
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com | |
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com | |
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com | |
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com | |
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com | |
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com | |
| Phone | +1(800)492-4515 |
| customerservice@budgethearingaids.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00810068832730 [Primary] |
FDA Product Code
| QUG | Hearing Aid, Air-Conduction with Wireless Technology, Over the Counter |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2025-03-31 |
| Device Publish Date | 2025-03-21 |
On-Brand Devices [Lucid Hearing®]
| 00810068832761 | BHA-Engage Rech-OTC-A-BG |
| 00810068832754 | BHA-Engage Rech-OTC-I-BG |
| 00810068832747 | BHA-Engage-OTC-A-BG |
| 00810068832730 | BHA-Engage-OTC-I-BG |
| 00810068832976 | BHA-Engage Rech-OTC-A-GR |
| 00810068832969 | BHA-Engage Rech-OTC-I-GR |
| 00810068832952 | BHA-Engage Rech-OTC-A-BK |
| 00810068832945 | BHA-Engage Rech-OTC-I-BK |
| 00810068832938 | BHA-Engage-OTC-A-GR Right Monaural |
| 00810068832921 | BHA-Engage-OTC-A-GR Left Monaural |
| 00810068832914 | BHA-Engage-OTC-I-GR Right Monaural |
| 00810068832907 | BHA-Engage-OTC-I-GR Left Monaural |
| 00810068832891 | BHA-Engage-OTC-A-BK Right Monaural |
| 00810068832884 | BHA-Engage-OTC-A-BK Left Monaural |
| 00810068832877 | BHA-Engage-OTC-I-BK Right Monaural |
| 00810068832860 | BHA-Engage-OTC-I-BK Left Monaural |
| 00810068832853 | BHA-Engage-OTC-A-BG Right Monaural |
| 00810068832846 | BHA-Engage-OTC-A-BG Left Monaural |
| 00810068832839 | BHA-Engage-OTC-I-BG Right Monaural |
| 00810068832822 | BHA-Engage-OTC-I-BG Left Monaural |
| 00810068832815 | BHA-Engage-OTC-A-GR |
| 00810068832808 | BHA-Engage-OTC-I-GR |
| 00810068832792 | BHA-Engage-OTC-A-BK |
| 00810068832785 | BHA-Engage-OTC-I-BK |
| 00810068832778 | BHA-FIO |
| 00810068832327 | U104BT Silver Hearing Aid |
| 00810068832310 | A104BT Silver Hearing Aid |
| 00810068832303 | U64BT Brown Hearing Aid |
| 00810068832297 | A64BT Brown Hearing Aid |
| 00810068832280 | U64BT Black Hearing Aid |
| 00810068832273 | A64BT Black Hearing Aid |
| 00810068832266 | U64BT Grey Hearing Aid |
| 00810068832259 | A64BT Grey Hearing Aid |
| 00810068832242 | U64BT Beige Hearing Aid |
| 00810068832235 | A64BT Beige Hearing Aid |
| 00810068832228 | U128BT Silver Hearing Aid |
| 00810068832211 | A128BT Silver Hearing Aid |
| 00810068832204 | U32BT Brown Hearing Aid |
| 00810068832198 | A32BT Brown Hearing Aid |
| 00810068832181 | U32BT Black Hearing Aid |
| 00810068832174 | A32BT Black Hearing Aid |
| 00810068832167 | U32BT Grey Hearing Aid |
| 00810068832150 | A32BT Grey Hearing Aid |
| 00810068832143 | U32BT Beige Hearing Aid |
| 00810068832136 | A32BT Beige Hearing Aid |
Trademark Results [Lucid Hearing]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
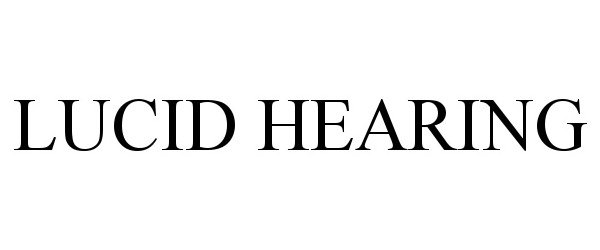 LUCID HEARING 97377206 not registered Live/Pending |
Team IP Holdings, LLC 2022-04-22 |
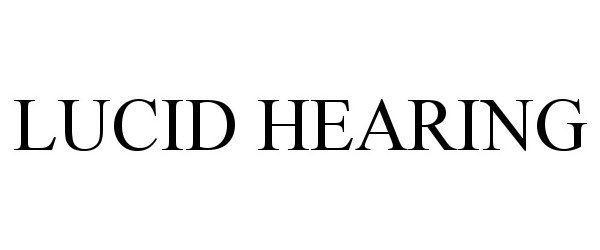 LUCID HEARING 90534033 not registered Live/Pending |
Team IP Holdings, LLC 2021-02-18 |
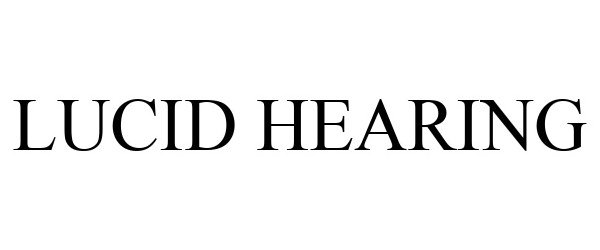 LUCID HEARING 90002207 not registered Live/Pending |
Team IP Holdings, LLC 2020-06-15 |
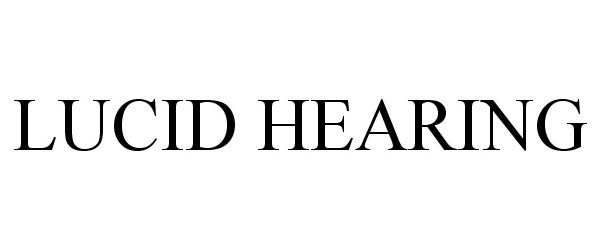 LUCID HEARING 87018083 5292120 Live/Registered |
TEAM IP HOLDINGS, LLC 2016-04-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.