BioSkin
GUDID 00810175570099
Velvet blue PF 12" nitrile glove, 10bx/cs
LIBERTY GLOVE & SAFETY, LLC
Nitrile examination/treatment glove, non-powdered, non-antimicrobial| Primary Device ID | 00810175570099 |
| NIH Device Record Key | dab1e4f7-cd6c-4f90-a9b7-48c6d6631975 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | BioSkin |
| Version Model Number | 2013MLXXL |
| Company DUNS | 111313244 |
| Company Name | LIBERTY GLOVE & SAFETY, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00810175570099 [Primary] |
FDA Product Code
| LZA | Polymer Patient Examination Glove |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-08-30 |
| Device Publish Date | 2024-08-22 |
On-Brand Devices [BioSkin]
| 00850017044319 | Blue Latex high-risk examination glove, 50pcs/bx |
| 00850017044395 | Blue Latex high-risk examination glove, 50pcs/bx |
| 00850017044371 | Blue Latex high-risk examination glove, 50pcs/bx |
| 00850017044357 | Blue Latex high-risk examination glove, 50pcs/bx |
| 00850017044333 | Blue Latex high-risk examination glove, 50pcs/bx |
| 00850017044326 | Blue Latex high-risk examination glove, 10bx/cs |
| 00850017044401 | Blue Latex high-risk examination glove, 10bx/cs |
| 00850017044388 | Blue Latex high-risk examination glove, 10bx/cs |
| 00850017044364 | Blue Latex high-risk examination glove, 10bx/cs |
| 00850017044340 | Blue Latex high-risk examination glove, 10bx/cs |
| 00850017044999 | Black PF nitrile glove, 10bx/cs |
| 00850017044982 | Black PF nitrile glove, 100pcs/bx |
| 00850017044975 | Black PF nitrile glove, 10bx/cs |
| 00850017044968 | Black PF nitrile glove, 100pcs/bx |
| 00850017044951 | Black PF nitrile glove, 10bx/cs |
| 00850017044944 | Black PF nitrile glove, 100pcs/bx |
| 00850017044937 | Black PF nitrile glove, 10bx/cs |
| 00850017044920 | Black PF nitrile glove, 100pcs/bx |
| 00850017044913 | White PF latex glove, 10bx/cs |
| 00850017044906 | White PF latex glove, 100pcs/bx |
| 00850017044890 | White PF latex glove, 10bx/cs |
| 00850017044883 | White PF latex glove, 100pcs/bx |
| 00850017044876 | White PF latex glove, 10bx/cs |
| 00850017044869 | White PF latex glove, 100pcs/bx |
| 00850017044852 | White PF latex glove, 10bx/cs |
| 00850017044845 | White PF latex glove, 100pcs/bx |
| 00850017044838 | White PF latex glove, 10bx/cs |
| 00850017044821 | White PF latex glove, 100pcs/bx |
| 00850017044630 | Black PF nitrile glove, 10bx/cs |
| 00850017044593 | Black PF nitrile glove, 100pcs/bx |
| 00810175570143 | Velvet blue PF 12" nitrile glove, 10bx/cs |
| 00810175570099 | Velvet blue PF 12" nitrile glove, 10bx/cs |
| 00810175570082 | Velvet blue PF 12" nitrile glove, 10bx/cs |
| 00810175570075 | Velvet blue PF 12" nitrile glove, 10bx/cs |
| 00810175570051 | Velvet blue PF 12" nitrile glove, 10bx/cs |
| 00810175570044 | Velvet blue PF 12" nitrile glove, 100pc/bx |
| 00810175570037 | Velvet blue PF 12" nitrile glove, 100pc/bx |
| 00810175570020 | Velvet blue PF 12" nitrile glove, 100pc/bx |
| 00810175570013 | Velvet blue PF 12" nitrile glove, 100pc/bx |
| 00810175570006 | Velvet blue PF 12" nitrile glove, 100pc/bx |
| 10850017044491 | Black PF nitrile glove, 10bx/cs |
| 10850017044484 | Black PF nitrile glove, 10bx/cs |
| 00850017044531 | Black PF nitrile glove, 10bx/cs |
| 00850017044524 | Black PF nitrile glove, 100pcs/bx |
| 00850017044517 | Black PF nitrile glove, 10bx/cs |
| 00850017044500 | Black PF nitrile glove, 100pcs/bx |
| 00850017044494 | Black PF nitrile glove, 100pcs/bx |
| 00850017044487 | Black PF nitrile glove, 100pcs/bx |
| 00850017044302 | White PF latex glove, 10bx/cs |
| 00850017044296 | White PF latex glove, 100pcs/bx |
Trademark Results [BioSkin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BIOSKIN 98745709 not registered Live/Pending |
Cardiai Technologies 2024-09-11 |
 BIOSKIN 90323698 not registered Live/Pending |
Curve Toys, LLC 2020-11-17 |
 BIOSKIN 88141704 not registered Live/Pending |
Curve Toys, LLC 2018-10-03 |
 BIOSKIN 87602157 5447763 Live/Registered |
Cropper Medical, Inc. 2017-09-09 |
 BIOSKIN 87602111 5583791 Live/Registered |
Cropper Medical, Inc. 2017-09-09 |
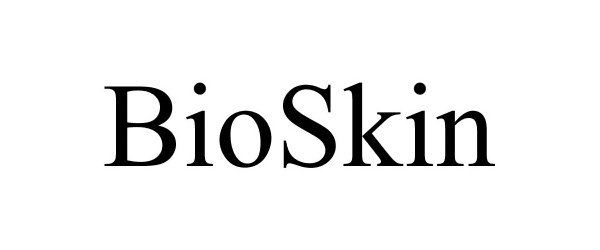 BIOSKIN 86785159 4910571 Live/Registered |
Human Regenerative Technologies LLC 2015-10-12 |
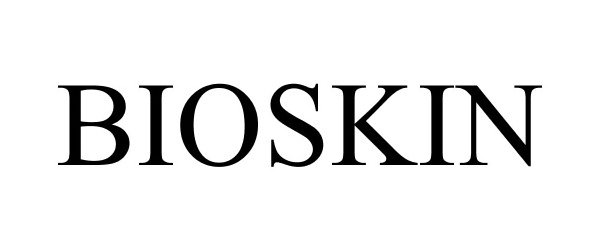 BIOSKIN 86521470 5595929 Live/Registered |
Salcura Limited 2015-02-02 |
 BIOSKIN 78288650 2871779 Live/Registered |
LIBERTY GLOVE & SAFETY, LLC 2003-08-18 |
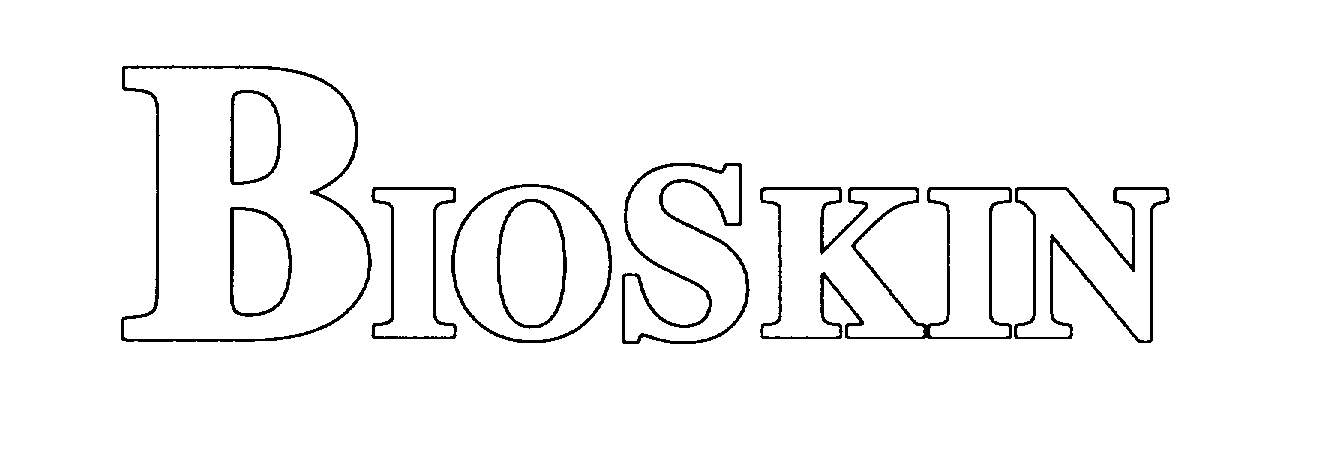 BIOSKIN 78008354 not registered Dead/Abandoned |
Liberty Glove, Inc. 2000-05-16 |
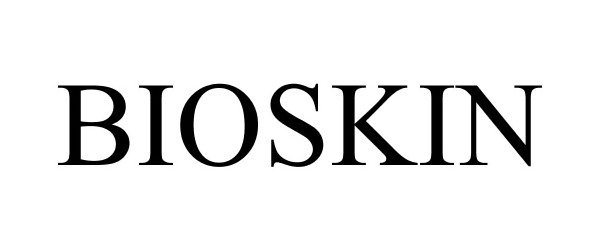 BIOSKIN 77079897 3413976 Dead/Cancelled |
Fidia Farmaceutici S.p.A. 2007-01-10 |
 BIOSKIN 75792598 not registered Dead/Abandoned |
Fidia S.p.a. 1999-09-03 |
 BIOSKIN 75519451 2434510 Dead/Cancelled |
FIDIA S.p.A. 1998-07-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.