SmartLyte® Plus Electrolyte Analyzer Na+/K+/Cl-/Ca++/Li+
GUDID 00811403010028
The SmartLyte® Plus Na+, K+, Cl-, Ca++, Li+ Electrolyte Analyzer is a microprocessor-controlled analyzers which utilize ion-selective electrodes for the measurement of sodium, potassium, chloride, calcium and lithium in serum, plasma, whole blood, pre-diluted urine samples, and QC materials. The analyzer self-calibrates using Diamond Diagnostics Fluid Pack every 4 hours throughout the day or on request. Sodium, potassium, chloride and calcium are commonly measured for use in the diagnosis and management of patients with a broad range of renal, metabolic and cardiovascular disorders. Lithium is a drug used to treat mental illness.
Diamond Diagnostics Inc.
Ion-selective analyser IVD| Primary Device ID | 00811403010028 |
| NIH Device Record Key | 59e64393-97ea-4ed0-b9ac-deb21aeec548 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | SmartLyte® Plus Electrolyte Analyzer Na+/K+/Cl-/Ca++/Li+ |
| Version Model Number | 0-DD-SMARTLYTEPLUS |
| Company DUNS | 947514303 |
| Company Name | Diamond Diagnostics Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00811403010028 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K200544
FDA Product Code
| JJE | Analyzer, Chemistry (Photometric, Discrete), For Clinical Use |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-06-13 |
| Device Publish Date | 2023-06-05 |
Devices Manufactured by Diamond Diagnostics Inc.
| 00811403010158 - Photometer Lamp | 2023-06-21 |
| 00811403010240 - Reference Solution | 2023-06-21 |
| 00811403013074 - Pump Tubing Kit | 2023-06-21 |
| 00811403013173 - C1/C2 Cleaning Solution | 2023-06-21 |
| 00811403010387 - Wash Pack | 2023-06-21 |
| 00811403013296 - pH Electrode | 2023-06-21 |
| 00811403013302 - Calcium Electrode | 2023-06-21 |
| 00811403013319 - Potassium Electrode | 2023-06-21 |
Trademark Results [SmartLyte]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
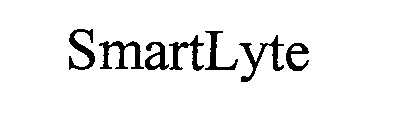 SMARTLYTE 76698012 3848109 Live/Registered |
Diamond Diagnostics Inc. 2009-06-22 |
 SMARTLYTE 76504618 3140826 Dead/Cancelled |
Acolyte Technologies Corporation 2003-04-08 |
 SMARTLYTE 76063793 not registered Dead/Abandoned |
Acolyte Systems Inc. 2000-06-02 |
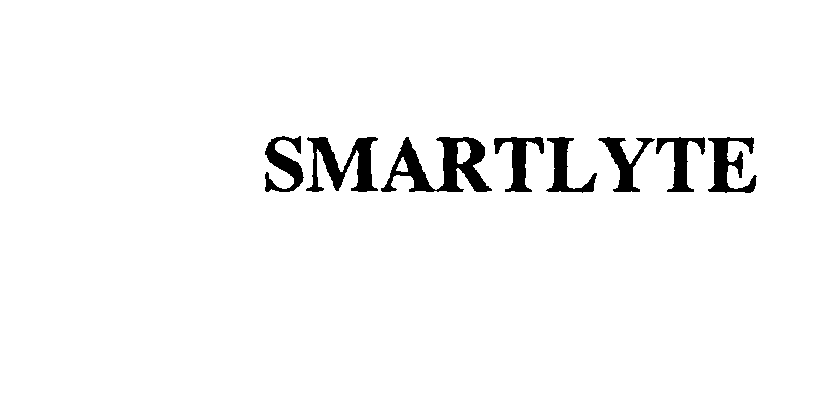 SMARTLYTE 76063792 not registered Dead/Abandoned |
Acolyte Systems Inc. 2000-06-02 |
 SMARTLYTE 74663526 not registered Dead/Abandoned |
IQ International, Inc. 1995-04-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.