Osseofit™ Porous Tissue Matrix 50604-10
GUDID 00812337021548
DSM BIOMEDICAL, INC.
Composite bone graft| Primary Device ID | 00812337021548 |
| NIH Device Record Key | e6c2c8ae-7832-4798-9ac8-1f14eafaaab1 |
| Commercial Distribution Discontinuation | 2019-09-30 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | Osseofit™ Porous Tissue Matrix |
| Version Model Number | 50604-10 |
| Catalog Number | 50604-10 |
| Company DUNS | 131107757 |
| Company Name | DSM BIOMEDICAL, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
| Circumference | 10 Millimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00812337021548 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K060917
FDA Product Code
| MQV | Filler, Bone Void, Calcium Compound |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 5 |
| Public Version Date | 2019-10-01 |
| Device Publish Date | 2015-09-01 |
On-Brand Devices [Osseofit™ Porous Tissue Matrix]
| 00812337021562 | 50604-15 |
| 00812337021555 | 50604-12 |
| 00812337021548 | 50604-10 |
| 00812337021531 | 50604-08 |
| 00812337021524 | 50604-06 |
| 00812337021517 | 50604-04 |
Trademark Results [Osseofit]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
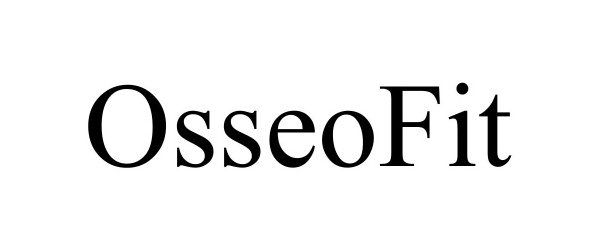 OSSEOFIT 97924878 not registered Live/Pending |
Zimmer, Inc. 2023-05-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.