IS-2000
GUDID 00813277020127
Impression Material, Ultra Lite Body Regular
ADA PRODUCTS CO, INC
Silicone dental impression material| Primary Device ID | 00813277020127 |
| NIH Device Record Key | 1caec6b0-440c-4095-8342-7bf7b899b2af |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | IS-2000 |
| Version Model Number | 40100 |
| Company DUNS | 624504028 |
| Company Name | ADA PRODUCTS CO, INC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 877-243-7711 |
| info@adaproducts.net | |
| Phone | 877-243-7711 |
| info@adaproducts.net |
Operating and Storage Conditions
| Storage Environment Temperature | Between 46 Degrees Fahrenheit and 75 Degrees Fahrenheit |
| Storage Environment Temperature | Between 46 Degrees Fahrenheit and 75 Degrees Fahrenheit |
| Storage Environment Temperature | Between 46 Degrees Fahrenheit and 75 Degrees Fahrenheit |
| Storage Environment Temperature | Between 46 Degrees Fahrenheit and 75 Degrees Fahrenheit |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00813277020127 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K951075
FDA Product Code
| ELW | Material, Impression |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2017-04-10 |
On-Brand Devices [IS-2000]
| 00813277020288 | Impression Material, Heavy Body Fast, Bulk |
| 00813277020271 | Impression Material, Heavy Body Regular, Bulk |
| 00813277020264 | Impression Material, Monophase Regular, Bulk |
| 00813277020257 | Impression Material, Lite Body Fast, Bulk |
| 00813277020240 | Impression Material, Lite Body Regular, Bulk |
| 00813277020233 | Impression Material, Ultra Lite Body Regular, Bulk |
| 00813277020226 | Bite Registration Material, Fast Set |
| 00813277020196 | Impression Material, Putty Kit (Base & Catalyst) |
| 00813277020189 | Impression Material, Maximum Monophase Regular |
| 00813277020172 | Impression Material, Heavy Body Fast |
| 00813277020165 | Impression Material, Heavy Body Regular |
| 00813277020158 | Impression Material, Monophase Regular |
| 00813277020141 | Impression Material, Lite Body Fast |
| 00813277020134 | Impression Material, Lite Body Regular |
| 00813277020127 | Impression Material, Ultra Lite Body Regular |
Trademark Results [IS-2000]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IS-2000 75404779 2225656 Dead/Cancelled |
Cognex Corporation 1997-12-12 |
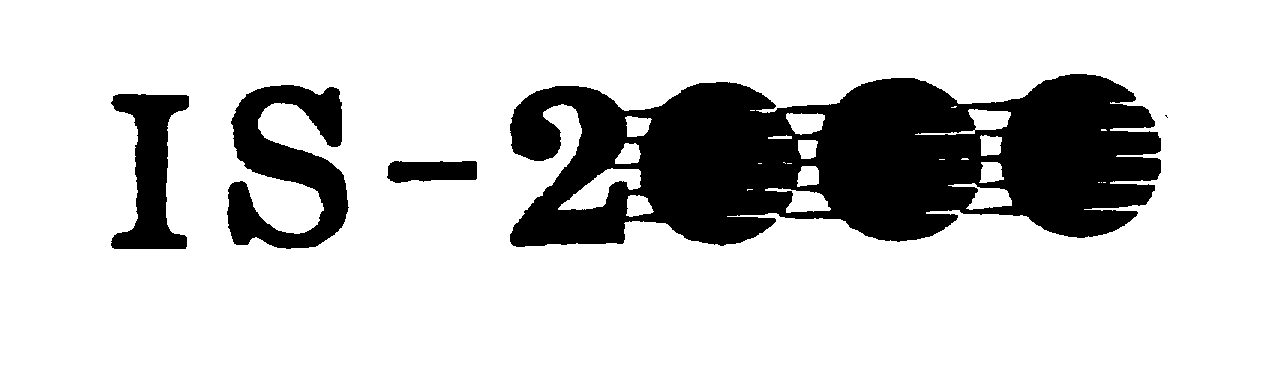 IS-2000 74370579 1893210 Dead/Cancelled |
Impact Solutions, Inc. 1993-03-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.