Simile
GUDID 00814978021864
Universal Composite; Syringe; Universal Opaque
Kerr Corporation
Light-cured dental restorative composite resin| Primary Device ID | 00814978021864 |
| NIH Device Record Key | f863ba33-fc0b-47e8-a7fd-10bbd2898483 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Simile |
| Version Model Number | N56AI |
| Company DUNS | 199354556 |
| Company Name | Kerr Corporation |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com | |
| Phone | +18005377123 |
| customercare@kavokerrgroup.com |
Device Dimensions
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
| Weight | 4 Gram |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Store at ambient temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store at ambient temperature |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 28 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00814978021864 [Primary] |
FDA Product Code
| EBF | MATERIAL, TOOTH SHADE, RESIN |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2018-03-29 |
| Device Publish Date | 2016-08-01 |
On-Brand Devices [Simile]
| 00814978023271 | Universal Composite; 10 Shade Single Dose Kit; Opaque Universal, Clear Incisal |
| 00814978023264 | Universal Composite; 10 Shade Syringe Kit; Opaque Universal, Clear Incisal |
| 00814978021970 | Universal Composite; Syringe; B0 |
| 00814978021963 | Universal Composite; Syringe; A0 |
| 00814978021956 | Universal Composite; Syringe; D4 |
| 00814978021949 | Universal Composite; Syringe; D3 |
| 00814978021932 | Universal Composite; Syringe; D2 |
| 00814978021925 | Universal Composite; Syringe; C4 |
| 00814978021918 | Universal Composite; Syringe; B4 |
| 00814978021901 | Universal Composite; Syringe; A4 |
| 00814978021895 | Universal Composite; Syringe; C1 |
| 00814978021888 | Universal Composite; Syringe; C3 |
| 00814978021871 | Nano-Hybrid Composite; Syringe; Clear Incisal |
| 00814978021864 | Universal Composite; Syringe; Universal Opaque |
| 00814978021857 | Universal Composite; Syringe; C2 |
| 00814978021840 | Universal Composite; Syringe; B3 |
| 00814978021833 | Universal Composite; Syringe; B2 |
| 00814978021826 | Universal Composite; Syringe; B1 |
| 00814978021819 | Universal Composite; Syringe; A3.5 |
| 00814978021802 | Universal Composite; Syringe; A3 |
| 00814978021796 | Universal Composite; Syringe; A2 |
| 00814978021789 | Universal Composite; Syringe; A1 |
| 00841396181344 | Universal Composite; Syringe; Opaque Dark |
| 00841396181337 | Universal Composite; Syringe; Opaque Medium |
| 10814978022172 | Universal Composite; Single Doses; B0 |
| 10814978022165 | Universal Composite; Single Doses; A0 |
| 10814978022158 | Universal Composite; Single Doses; D4 |
| 10814978022141 | Universal Composite; Single Doses; D3 |
| 10814978022134 | Universal Composite; Single Doses; D2 |
| 10814978022127 | Universal Composite; Single Doses; C4 |
| 10814978022110 | Universal Composite; Single Doses; B4 |
| 10814978022103 | Universal Composite; Single Doses; A4 |
| 10814978022097 | Nano-Hybrid Composite; Single Doses; Clear Incisal |
| 10814978022080 | Universal Composite; Single Doses; Universal Opaque |
| 10814978022073 | Universal Composite; Single Doses; C3 |
| 10814978022066 | Universal Composite; Single Doses; C2 |
| 10814978022059 | Universal Composite; Single Doses; C1 |
| 10814978022042 | Universal Composite; Single Doses; B3 |
| 10814978022035 | Universal Composite; Single Doses; B2 |
| 10814978022028 | Universal Composite; Single Doses; B1 |
| 10814978022011 | Universal Composite; Single Doses; A3.5 |
| 10814978022004 | Universal Composite; Single Doses; A3 |
| 10814978021991 | Universal Composite; Single Doses; A2 |
| 10814978021984 | Universal Composite; Single Doses; A1 |
Trademark Results [Simile]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SIMILE 98103246 not registered Live/Pending |
CLT Logistics, Inc. 2023-07-26 |
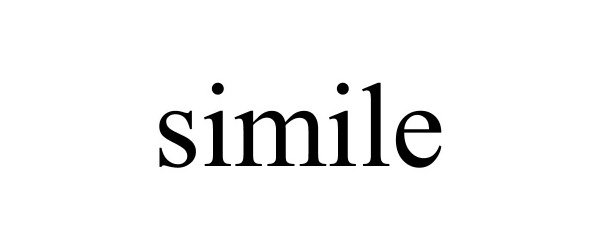 SIMILE 90808181 not registered Live/Pending |
SHENZHEN SUNGWORLD ELECTRONICS CO LIMITED 2021-07-02 |
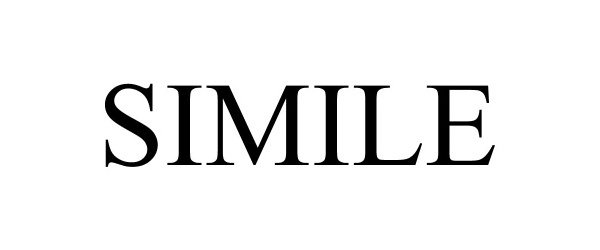 SIMILE 90552702 not registered Live/Pending |
SPK PRODUCTIONS, LLC 2021-03-01 |
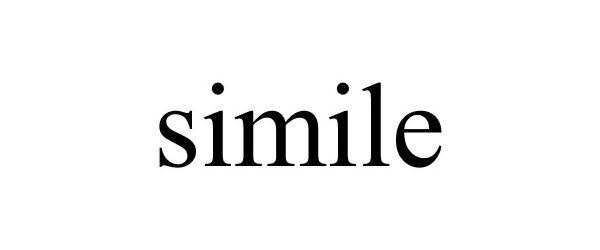 SIMILE 88539325 not registered Live/Pending |
Guangzhou Siwang Lai Electronic Technology Co., Ltd. 2019-07-26 |
 SIMILE 87042166 not registered Live/Pending |
Depth Kit Inc. 2016-05-18 |
 SIMILE 85514012 4194462 Live/Registered |
Pentron Clinical Technologies, LLC 2012-01-11 |
 SIMILE 78767714 3735340 Dead/Cancelled |
Fruit of the Earth, Inc. 2005-12-06 |
 SIMILE 78132714 2785052 Dead/Cancelled |
Jeneric/Pentron, Incorporated 2002-06-03 |
 SIMILE 75936734 not registered Dead/Abandoned |
Cumran, Inc. 2000-03-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.