V-SPOT
GUDID 00815137020452
Nipple Markers, 2.3mm non-metallic pellet
Beekley Corporation
Radiographic image marker, manual| Primary Device ID | 00815137020452 |
| NIH Device Record Key | 8c17521c-c274-4bfa-8ec5-6c51a11ff683 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | V-SPOT |
| Version Model Number | 703 |
| Company DUNS | 001157841 |
| Company Name | Beekley Corporation |
| Device Count | 130 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00815137020452 [Primary] |
| GS1 | 10815137020459 [Unit of Use] |
FDA Product Code
| JAC | System, X-Ray, Film Marking, Radiographic |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2019-07-30 |
| Device Publish Date | 2019-07-22 |
On-Brand Devices [V-SPOT]
| 00815137021848 | Nipple Markers, 2.3mm non-metallic pellet |
| 00815137021701 | Nipple Markers, 2.5mm lead-free pellet |
| 00815137020452 | Nipple Markers, 2.3mm non-metallic pellet |
| 00815137020315 | Nipple Markers, 2.5mm lead-free pellet |
Trademark Results [V-SPOT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 V-SPOT 98829422 not registered Live/Pending |
GINKGO MEDIA GROUP CO., LIMITED 2024-10-30 |
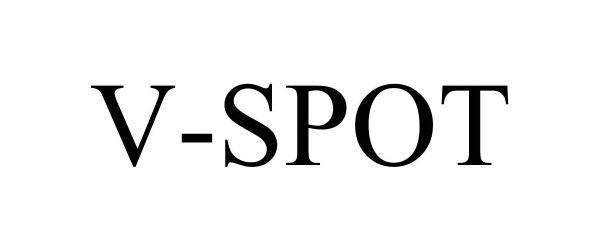 V-SPOT 90536809 not registered Live/Pending |
Beekley Corporation 2021-02-19 |
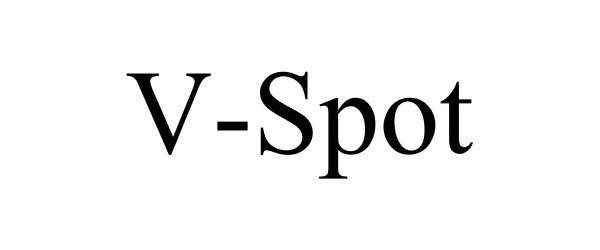 V-SPOT 86422114 not registered Dead/Abandoned |
V Spot, LLC 2014-10-13 |
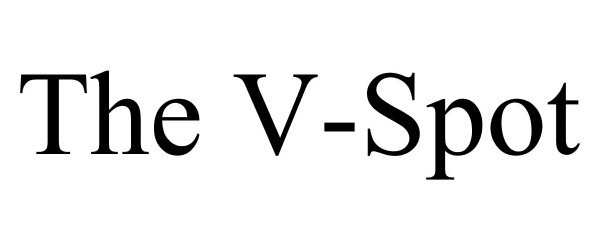 V-SPOT 86199501 4616911 Live/Registered |
V Spot, LLC 2014-02-20 |
 V-SPOT 85076362 3973122 Live/Registered |
V-Day Corporation 2010-07-01 |
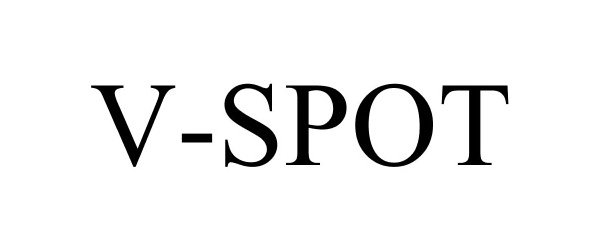 V-SPOT 78733096 not registered Dead/Abandoned |
Victoria's Secret Stores Brand Management, Inc. 2005-10-14 |
 V-SPOT 76213557 not registered Dead/Abandoned |
HIJ, LLC 2001-02-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.