DUPLOSPRAY
GUDID 00815634020658
DUPLOSPRAY MIS APPLICATOR, 40CM (Gas Assist 1:1 used with Tisseel)
MICROMEDICS, INC.
Open-surgery adhesive/sealant applicator, dual-channel| Primary Device ID | 00815634020658 |
| NIH Device Record Key | cddf936d-5c39-48ea-8536-dabdcb38e109 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | DUPLOSPRAY |
| Version Model Number | 0600030 |
| Company DUNS | 066839630 |
| Company Name | MICROMEDICS, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00815634020658 [Primary] |
| GS1 | 10815634020655 [Package] Package: SHELF BOX [5 Units] In Commercial Distribution |
| GS1 | 20815634020652 [Package] Contains: 10815634020655 Package: SHIP BOX [1 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K061092
FDA Product Code
| FMF | Syringe, Piston |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-09-25 |
On-Brand Devices [DUPLOSPRAY]
| 00815634020665 | DUPLOSPRAY MIS APPLICATOR, 20CM (Gas Assist 1:1 used with Tisseel) |
| 00815634020658 | DUPLOSPRAY MIS APPLICATOR, 40CM (Gas Assist 1:1 used with Tisseel) |
| 00815634020641 | DUPLOSPRAY MIS APPLICATOR, 30CM (Gas Assist 1:1 used with Tisseel) |
| 20815634020584 | DuploSpray MIS Applicator with Snap Lock, 30cm |
| 20815634020577 | DuploSpray MIS Applicator with Snap Lock, 20cm |
| 20815634020492 | DUPLOSPRAY MIS APPLICATOR, 20CM (Gas Assist 1:1 used with Tisseel) |
| 20815634020485 | DUPLOSPRAY MIS APPLICATOR, 40CM (Gas Assist 1:1 used with Tisseel) |
| 20815634020478 | DUPLOSPRAY MIS APPLICATOR, 30CM (Gas Assist 1:1 used with Tisseel) |
| 00815634020214 | DUPLOSPRAY REPLACEMENT TIPS (Tip Alignment Tool with 2 Spray Tips per pouch) |
| 00815634020856 | DUPLOSPRAY MIS REGULATOR 1.5 BAR B11 |
| 10815634020594 | DuploSpray MIS Applicator with Snap Lock 40cm |
Trademark Results [DUPLOSPRAY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
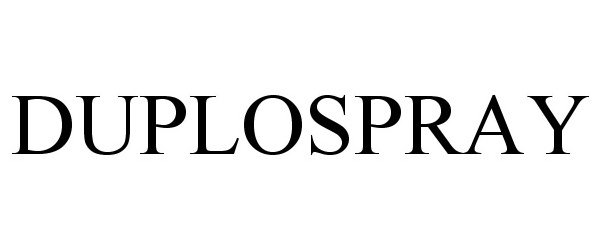 DUPLOSPRAY 78832246 3403357 Live/Registered |
Baxter International Inc. 2006-03-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.