REAL y-Series
GUDID 00815948029460
REAL y-Series 2
PENUMBRA, INC.
Mental health/function therapeutic software, virtual reality| Primary Device ID | 00815948029460 |
| NIH Device Record Key | 7059dfb3-5d69-4ecf-8bee-05769abdc285 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | REAL y-Series |
| Version Model Number | RLY2-A |
| Company DUNS | 191077671 |
| Company Name | PENUMBRA, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00815948029460 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K183296
FDA Product Code
| ISD | Exerciser, Measuring |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-04-24 |
| Device Publish Date | 2024-04-16 |
On-Brand Devices [REAL y-Series]
| 00815948029484 | REAL y-Series GO |
| 00815948029477 | REAL y-Series 2 Eval |
| 00815948029460 | REAL y-Series 2 |
Trademark Results [REAL y-Series]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
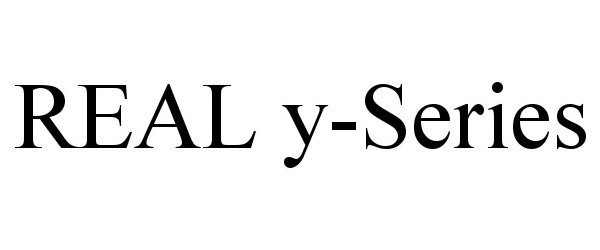 REAL Y-SERIES 90826053 not registered Live/Pending |
Penumbra, Inc. 2021-07-13 |
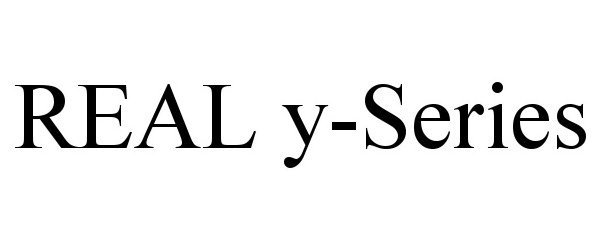 REAL Y-SERIES 90826048 not registered Live/Pending |
Penumbra, Inc. 2021-07-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.