CoolView
GUDID 00816722022301
CoolView Laser Handpiece
CUTERA, INC.
Dermatological frequency-doubled solid-state laser system| Primary Device ID | 00816722022301 |
| NIH Device Record Key | a77c7181-8b2d-41da-bad3-47530ea4f347 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | CoolView |
| Version Model Number | CoolView |
| Company DUNS | 041071643 |
| Company Name | CUTERA, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 1 415 657-5500 |
| info@cutera.com | |
| Phone | 1 415 657-5500 |
| info@cutera.com | |
| Phone | 1 415 657-5500 |
| info@cutera.com | |
| Phone | 1 415 657-5500 |
| info@cutera.com | |
| Phone | 1 415 657-5500 |
| info@cutera.com | |
| Phone | 1 415 657-5500 |
| info@cutera.com | |
| Phone | 1 415 657-5500 |
| info@cutera.com | |
| Phone | 1 415 657-5500 |
| info@cutera.com | |
| Phone | 1 415 657-5500 |
| info@cutera.com | |
| Phone | 1 415 657-5500 |
| info@cutera.com | |
| Phone | 1 415 657-5500 |
| info@cutera.com | |
| Phone | 1 415 657-5500 |
| info@cutera.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00816722022301 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K132185
FDA Product Code
| GEX | Powered Laser Surgical Instrument |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-09-23 |
On-Brand Devices [CoolView]
| 00816722022301 | CoolView Laser Handpiece |
| 00816722022325 | CoolView Laser Handpiece for EV+ (EV2) |
| 00816722022363 | CoolView Laser Handpiece for EV+ (EV3) |
Trademark Results [CoolView]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COOLVIEW 88079938 5732239 Live/Registered |
Lee Aerospace, Inc. 2018-08-15 |
 COOLVIEW 88079913 5732236 Live/Registered |
Lee Aerospace, Inc. 2018-08-15 |
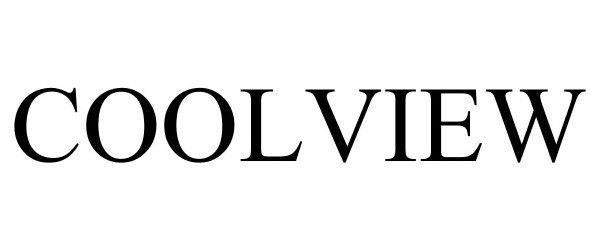 COOLVIEW 87534365 5500895 Live/Registered |
Zero Zone, Inc. 2017-07-19 |
 COOLVIEW 87054137 5230924 Live/Registered |
LG ELECTRONICS INC. 2016-05-31 |
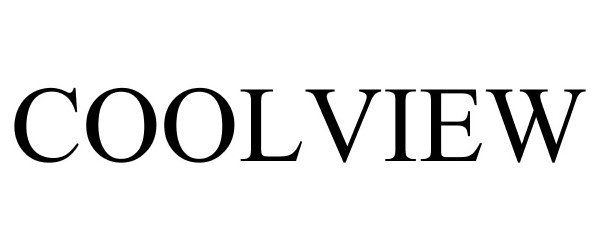 COOLVIEW 86186350 4609096 Live/Registered |
Hawkeye Distributing, LLC 2014-02-06 |
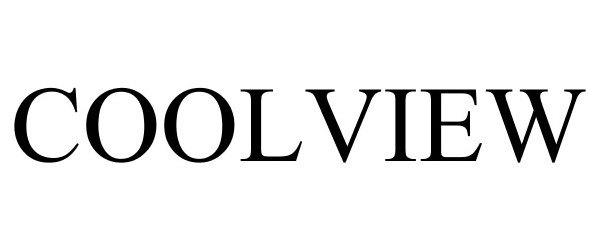 COOLVIEW 78820543 3180976 Dead/Cancelled |
Phoenix Manufacturing, Inc. 2006-02-22 |
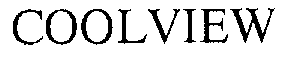 COOLVIEW 76307299 not registered Dead/Abandoned |
Cooley Group Holdings, Inc. 2001-08-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.