Onyx
GUDID 00817051020471
Onyx Etch 1.2ml 40% Phosphoric Acid Etching Gel, 15 syringes, 50 tips
CENTRIX, INC.
Dental etching solution| Primary Device ID | 00817051020471 |
| NIH Device Record Key | 04197dce-59ad-47f1-a108-6f03c7a4c7e8 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Onyx |
| Version Model Number | 310054 |
| Company DUNS | 053707303 |
| Company Name | CENTRIX, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00817051020471 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K890140
FDA Product Code
| EBF | Material, Tooth Shade, Resin |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-09-23 |
On-Brand Devices [Onyx]
| 00817051020488 | Onyx Etch 1.2ml 40% Phosphoric Acid Etching gel, 15 syringes, 100 tips |
| 00817051020471 | Onyx Etch 1.2ml 40% Phosphoric Acid Etching Gel, 15 syringes, 50 tips |
| 00817051020464 | Onyx Etch 1.2ml 40% Phosphoric Acid Etching Gel, 6 syringes |
| 00817051020457 | Onyx Etch 1.2ml 40% Phosphoric Acid Etching Gel, 25 syringes |
Trademark Results [Onyx]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ONYX 98828730 not registered Live/Pending |
ONYX Motors Inc. 2024-10-30 |
 ONYX 98801511 not registered Live/Pending |
Avolin, LLC 2024-10-15 |
 ONYX 98785638 not registered Live/Pending |
Onyx Renewable Partners L.P. 2024-10-04 |
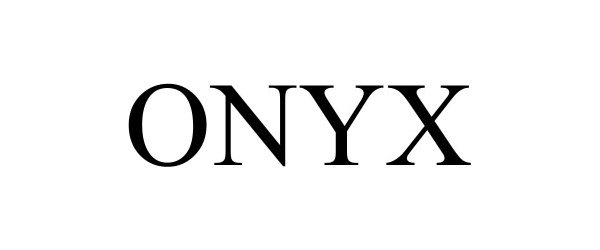 ONYX 98685416 not registered Live/Pending |
Tsos Technologies, Inc. 2024-08-06 |
 ONYX 98660451 not registered Live/Pending |
Christianson Systems, Inc. 2024-07-22 |
 ONYX 98627921 not registered Live/Pending |
Scruggs, Fred 2024-07-01 |
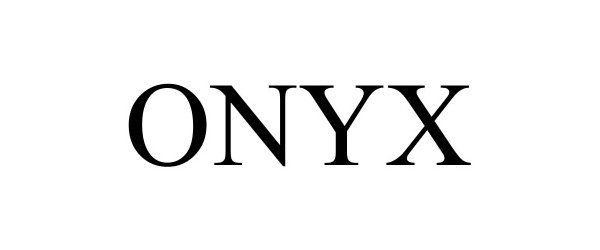 ONYX 98483781 not registered Live/Pending |
Raynor Mfg. Co. 2024-04-04 |
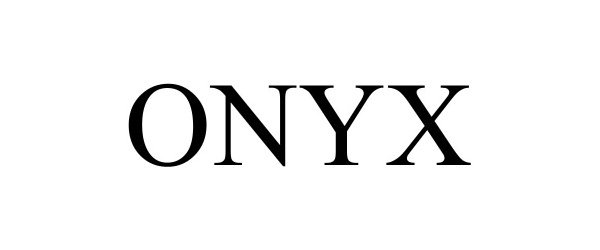 ONYX 98314783 not registered Live/Pending |
Nordson Corporation 2023-12-14 |
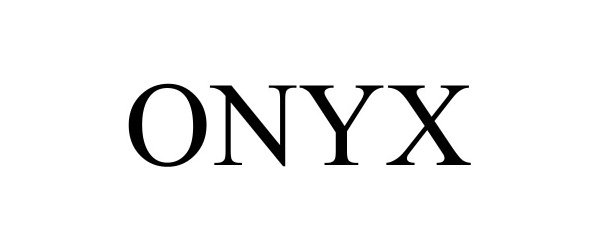 ONYX 98279338 not registered Live/Pending |
Christianson Systems, Inc. 2023-11-21 |
 ONYX 98271220 not registered Live/Pending |
KAPUR, BHARAT 2023-11-15 |
 ONYX 98180569 not registered Live/Pending |
Onyx Productions Inc. 2023-09-14 |
 ONYX 98155977 not registered Live/Pending |
21Shares AG 2023-08-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.