Dual Hose 30201
GUDID 00817369020620
Dual Hose - Nylon
NORMATEC INDUSTRIES LP
Sequential venous compression system| Primary Device ID | 00817369020620 |
| NIH Device Record Key | 3f39ed16-02f2-4155-be57-66015465dff8 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Dual Hose |
| Version Model Number | Dual Hose - Nylon |
| Catalog Number | 30201 |
| Company DUNS | 111448069 |
| Company Name | NORMATEC INDUSTRIES LP |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | true |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00817369020620 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K013436
FDA Product Code
| JOW | Sleeve, Limb, Compressible |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-09-22 |
Devices Manufactured by NORMATEC INDUSTRIES LP
| 00810050288743 - NormaTec | 2025-10-06 Normatec Elite Hip is a powered air pressure inflatable tube massager. It is intended to temporarily relieve minor muscle aches |
| 00810050281751 - NormaTec | 2025-08-04 NT3 Control Unit (only) |
| 00810050288026 - Hyperice / NormaTec | 2025-08-04 HYPERICE X2 KNEE |
| 00810050288033 - Hyperice / NormaTec | 2025-08-04 HYPERICE X2 SHOULDER |
| 00810050281713 - NormaTec | 2024-08-05 Normatec Hose Attachment |
| 00810050281812 - NormaTec | 2024-08-05 Normatec right arm attachment |
| 00810050286053 - NormaTec | 2024-05-31 Normatec Elite standard leg system is a powered air pressure inflatable tube massager. It is intended to temporarily relieve min |
| 00810050281997 - NormaTec | 2024-05-21 Normatec 3 is a powered air pressure inflatable tube massager. It is intended to temporarily relieve minor muscle aches and / o |
Trademark Results [Dual Hose]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
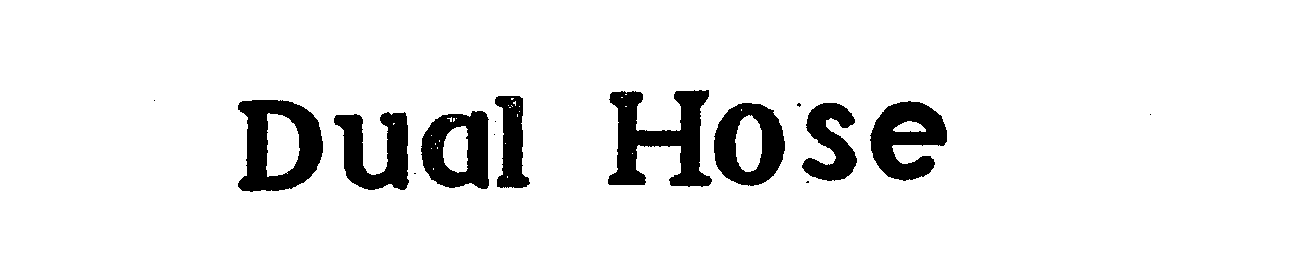 DUAL HOSE 71569710 0532258 Dead/Expired |
NATIONAL CYLINDER GAS COMPANY 1948-12-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.