iCup® DPG-167-1202
GUDID 00817405021345
iCup® E&I Urine Test 6 Panel Drug Screen Cup w/SVT (CR/pH/SG) (AMP1000/COC300/MET1000/OPI2000/OXY100/PCP25)
Instant Technologies, Inc.
Multiple drugs of abuse IVD, kit, immunochromatographic test (ICT), rapid| Primary Device ID | 00817405021345 |
| NIH Device Record Key | 0e9f05d9-3cbb-4962-8bbc-7aaa51df90fd |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | iCup® |
| Version Model Number | DPG-167-1202 |
| Catalog Number | DPG-167-1202 |
| Company DUNS | 019410187 |
| Company Name | Instant Technologies, Inc. |
| Device Count | 25 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com | |
| Phone | 1-800-340-4029 |
| Qa.Portsmouth@abbott.com |
Operating and Storage Conditions
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Handling Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00817405021345 [Unit of Use] |
| GS1 | 10817405021342 [Primary] |
FDA Product Code
| PUX | Test, Amphetamine, Employment And Insurance Testing, Exempt |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-09-28 |
| Device Publish Date | 2021-09-20 |
On-Brand Devices [iCup®]
| 10858945006338 | I-DUE-197-221 |
| 10833143000050 | One Step Multi-Drug Screen Test Card with the Integrated iCup® (CR/OX/pH)+ +++ |
| 10858945006321 | I-DUE-1107-291 |
| 10858945006314 | I-DUE-167-012 |
| 10858945006307 | I-DUE-167-342 |
| 10858945006291 | I-DUD-187-013 |
| 10858945006284 | I-DUE-1107-141 |
| 10858945006277 | I-DUD-1107-012 |
| 10858945006260 | I-DUD-197-014 |
| 10858945006253 | I-DUA-147-012 |
| 10858945006222 | i-DOA-197-041 |
| 10858945006215 | I-DUA-197-013 |
| 10858945006192 | I-DUA-157-115 |
| 10858945006185 | I-DUA-157-034 |
| 10858945006178 | I-DUE-187-071 |
| 10858945006154 | I-DUE-1127-022 |
| 10858945006147 | i-DUA-167-291 |
| 10858945006130 | I-DUA-167-022 |
| 10858945006123 | i-DOA-11107 |
| 10858945006116 | I-DUE-1117-101 |
| 10858945006109 | i-DOA-3137 |
| 10858945006093 | I-DUA-157-023 |
| 10858945006086 | I-DUA-167-012 |
| 10858945006079 | I-DUA-157-013 |
| 10858945006048 | I-DOA-1107-331 |
| 10858945006031 | I-DOA-1107-113 |
| 10858945006024 | I-DUE-187-241 |
| 10858945006017 | i-DOA-1237 |
| 10858945006000 | I-DOA-177-031 |
| 10833143000043 | One Step Multi-Drug Screen Test Card with the Integrated iCup® (Oxidants/Specific Gravity/pH)+ |
| 10833143000036 | One Step Multi-Drug Screen Test Card with the Integrated iCup® MTD>++(BZO/BAR)+(mAMP/OPI/P |
| 10858945006840 | A rapid, one step screening test for the simultaneous, qualitative detection of multiple drugs a |
| 10858945006208 | I-DUA-187-012 |
| 10858945006161 | I-DUE-147-241 |
| 10858945006062 | I-DOA-197-251 |
| 10858945006055 | I-DOA-1117-111 |
| 10858945006826 | I-DUD-177-461 |
| 10858945006819 | I-DUE-197-261 |
| 10858945006246 | I-DOA-1137-011 |
| 10858945006239 | i-DOA-1107-051 |
| 00817405021345 | iCup® E&I Urine Test 6 Panel Drug Screen Cup w/SVT (CR/pH/SG) (AMP1000/COC300/MET1000/OPI2000/O |
| 00817405021338 | iCup® E&I Urine Test 11 Panel Drug Screen Cup w/SVT (CR/pH/SG) (AMP1000/BAR300/BZO300/BUP10/COC |
Trademark Results [iCup]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
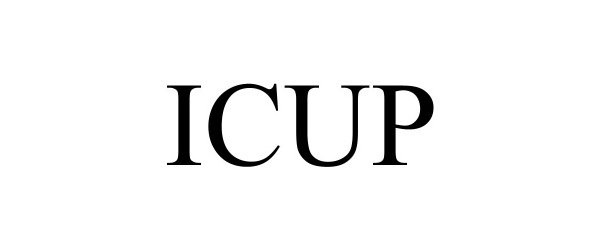 ICUP 97791787 not registered Live/Pending |
Aquarius Entertainment Merchandising Inc. 2023-02-13 |
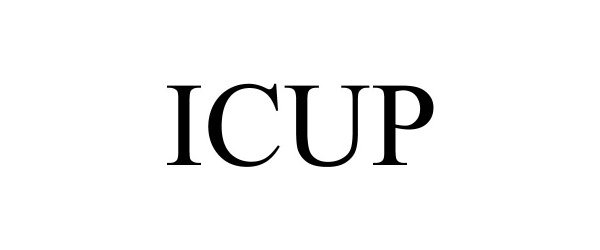 ICUP 88865667 not registered Live/Pending |
ICUP Inc. 2020-04-09 |
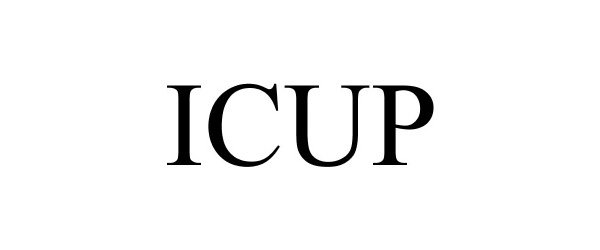 ICUP 88230234 not registered Live/Pending |
Max Bioscience 2018-12-14 |
 ICUP 87585683 5432994 Live/Registered |
Kyle Donovan 2017-08-28 |
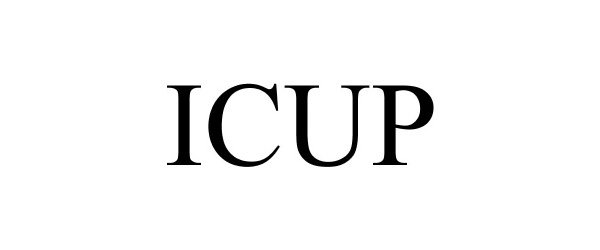 ICUP 85799542 not registered Dead/Abandoned |
ConforMIS, Inc. 2012-12-11 |
 ICUP 85765203 not registered Dead/Abandoned |
Donovan, Kyle 2012-10-26 |
 ICUP 85400330 4538158 Live/Registered |
COFFEE SOLUTIONS, LLC 2011-08-17 |
 ICUP 79070917 3811095 Dead/Cancelled |
LABORATOIRES GYNEAS 2009-04-09 |
 ICUP 78980298 3459782 Dead/Cancelled |
Intellectual Solutions, Inc. 2006-01-10 |
 ICUP 78788377 not registered Dead/Abandoned |
Intellectual Solutions, Inc. 2006-01-10 |
 ICUP 77655016 not registered Dead/Abandoned |
NLRS, LLC 2009-01-23 |
 ICUP 76490413 2866166 Live/Registered |
INSTANT TECHNOLOGIES, INC. 2003-02-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.