SPEEDLOCK OM-9758
GUDID 00817470001099
SPEEDLOCK PLUS KIT W/ MAGNUMWIRE (BLACK)
Smith & Nephew, Inc.
Tendon/ligament bone anchor, non-bioabsorbable| Primary Device ID | 00817470001099 |
| NIH Device Record Key | 59a85217-ffb0-4693-91aa-50108086e2ef |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | SPEEDLOCK |
| Version Model Number | OM-9758 |
| Catalog Number | OM-9758 |
| Company DUNS | 109903521 |
| Company Name | Smith & Nephew, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Safe |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00817470001099 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K130196
FDA Product Code
| MBI | FASTENER, FIXATION, NONDEGRADABLE, SOFT TISSUE |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 6 |
| Public Version Date | 2020-01-31 |
| Device Publish Date | 2015-09-21 |
On-Brand Devices [SPEEDLOCK]
| 00817470012101 | SPEEDLOCK CAPSLOCK ULTRA PROC KIT 70X8.6 |
| 00817470012095 | SPEEDLOCK CAPSLOCK ULTRA PROC KIT 55X8.2 |
| 00817470010558 | SPEEDLOCK |
| 00817470002881 | SPEEDLOCK DRILL GUIDE |
| 00817470001099 | SPEEDLOCK PLUS KIT W/ MAGNUMWIRE (BLACK) |
| 00817470000719 | LABRAFIX SPEEDLOCK PLUS IMPL |
| 00817470000672 | LABRAFIX SPEEDLOCK PLUS (WHITE) |
| 00885556724675 | SPEEDLOCK |
Trademark Results [SPEEDLOCK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
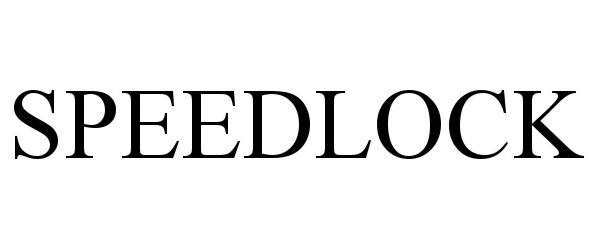 SPEEDLOCK 98210815 not registered Live/Pending |
#1 A LifeSafer, Inc. 2023-10-05 |
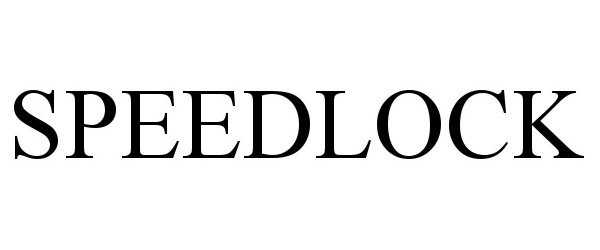 SPEEDLOCK 97343191 not registered Live/Pending |
ArthroCare Corporation 2022-04-01 |
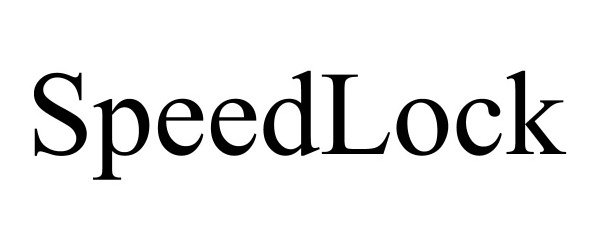 SPEEDLOCK 97341279 not registered Live/Pending |
PlayMakar Inc. 2022-03-31 |
 SPEEDLOCK 88132395 not registered Live/Pending |
Swarm Holdings LLC 2018-09-26 |
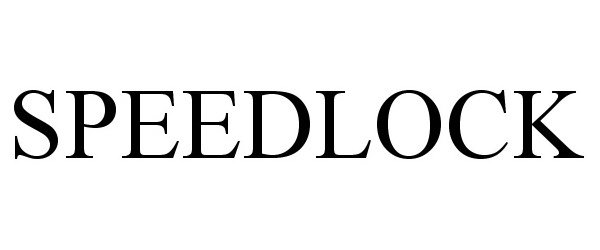 SPEEDLOCK 86975423 4646751 Live/Registered |
SHOCK DOCTOR, INC. 2013-08-07 |
 SPEEDLOCK 86031014 not registered Dead/Abandoned |
Nathan Sports Inc. 2013-08-07 |
 SPEEDLOCK 77849845 3881315 Dead/Cancelled |
ArthroCare Corporation 2009-10-15 |
 SPEEDLOCK 75694887 2727450 Dead/Cancelled |
Hafele America Co. 1999-04-30 |
 SPEEDLOCK 75293669 2237566 Dead/Cancelled |
VITTORIA INDUSTRIES LTD. 1997-05-19 |
 SPEEDLOCK 74714182 2064478 Dead/Cancelled |
SPEEDLOCK DUCTING PTY. LTD. 1995-08-11 |
 SPEEDLOCK 72064224 0683877 Live/Registered |
Waco Manufacturing Company 1958-12-12 |
 SPEEDLOCK 71537137 0503173 Dead/Expired |
E. J. BROOKS COMPANY 1947-10-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.