MAXO2+
GUDID 00817770020950
Analyzer with MicroMax BlenderBuddy 0-70 DISS
MAXTEC, LLC
Respiratory oxygen monitor, line-powered| Primary Device ID | 00817770020950 |
| NIH Device Record Key | 51b26342-7e11-475c-8420-963aebfff7ab |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | MAXO2+ |
| Version Model Number | R219P20-003 |
| Company DUNS | 169911828 |
| Company Name | MAXTEC, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00817770020950 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K040484
FDA Product Code
| CCL | Analyzer, Gas, Oxygen, Gaseous-Phase |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2019-01-14 |
| Device Publish Date | 2016-09-28 |
On-Brand Devices [MAXO2+]
| 00817770020967 | Analyzer with MicroMax BlenderBuddy 0-15 0-3.5 |
| 00817770020950 | Analyzer with MicroMax BlenderBuddy 0-70 DISS |
| 00817770020943 | Analyzer with Optiflow Jr. |
| 00817770020677 | MaxO2+A Analyzer with Adapter |
| 00817770020660 | Medical A International Analyzer |
Trademark Results [MAXO2+]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
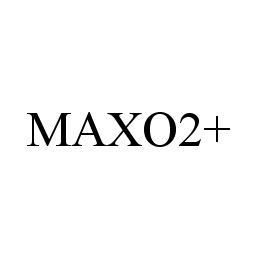 MAXO2+ 78340964 3069090 Live/Registered |
MAXTEC, LLC 2003-12-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.