SlimLine
GUDID 00821925010987
SlimLine Self-Dilating Autoclavable Operating Semi-Rigid Ureteroscope
Gyrus Acmi, Inc.
Rigid optical ureteroscope| Primary Device ID | 00821925010987 |
| NIH Device Record Key | 68d22288-b5c9-455c-acb2-48acfaedcfc1 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | SlimLine |
| Version Model Number | REXMRO-715A |
| Company DUNS | 007198742 |
| Company Name | Gyrus Acmi, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Operating and Storage Conditions
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
| Handling Environment Temperature | Between 15 Degrees Fahrenheit and 30 Degrees Fahrenheit |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00821925010987 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K012758
FDA Product Code
| FGB | Ureteroscope And Accessories, Flexible/Rigid |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-08-01 |
| Device Publish Date | 2024-07-24 |
On-Brand Devices [SlimLine]
| 00821925010987 | SlimLine Self-Dilating Autoclavable Operating Semi-Rigid Ureteroscope |
| 00821925009301 | SlimLine self-Dilating Autoclavable Operating Semi-Rigid Ureteroscope |
| 00821925002562 | Semi-Rigid Scissors 5 Fr, (1.67mm), 42 CM |
| 00821925002494 | Semi-Rigid Biopsy Forceps 5 Fr (1,67 mm), 42 CM |
| 00821925002470 | Semi-Rigid Alligator Grasping Forceps 5 Fr, (1.67mm), 42 CM |
Trademark Results [SlimLine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
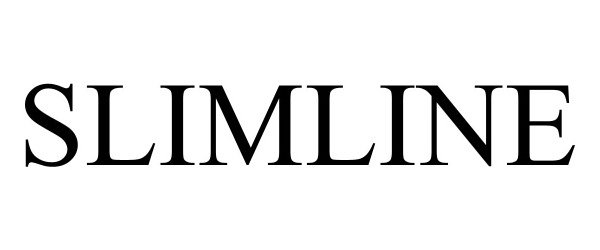 SLIMLINE 98641772 not registered Live/Pending |
Slimline LLC 2024-07-10 |
 SLIMLINE 98540331 not registered Live/Pending |
Elevation Spine 2024-05-08 |
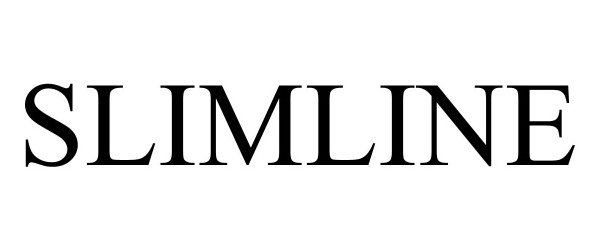 SLIMLINE 98375594 not registered Live/Pending |
Cabinet Hero Inc. 2024-01-25 |
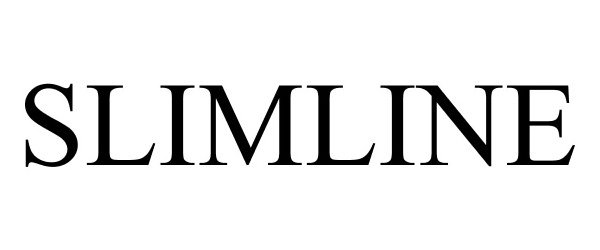 SLIMLINE 87912467 5622789 Live/Registered |
Bath Authority LLC 2018-05-08 |
 SLIMLINE 87254361 5236604 Live/Registered |
POETIC CASES INC. 2016-12-01 |
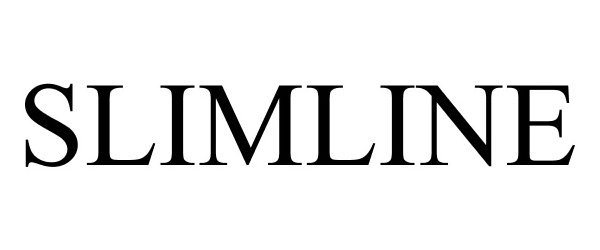 SLIMLINE 86751711 5456088 Live/Registered |
TECH TAC COMPANY, INC. 2015-09-09 |
 SLIMLINE 86600297 5409858 Live/Registered |
NOETIC TECHNOLOGIES INC. 2015-04-16 |
 SLIMLINE 86587039 4924266 Live/Registered |
Fairway Building Products, LLC 2015-04-03 |
 SLIMLINE 86551328 4930607 Live/Registered |
Shoals Technologies Group, LLC 2015-03-03 |
 SLIMLINE 86292011 not registered Dead/Abandoned |
JAMCO CORPORATION 2014-05-27 |
 SLIMLINE 85919307 not registered Dead/Abandoned |
Buck Knives, Inc. 2013-04-30 |
 SLIMLINE 85809252 4610439 Live/Registered |
Endocare, Inc. 2012-12-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.