Pivot Bipolar
GUDID 00822409015429
28x58mm Bipolar
Ortho Development Corporation
Femoral head bipolar component| Primary Device ID | 00822409015429 |
| NIH Device Record Key | 04ae14ba-75cb-486e-9a86-0fe278da4eae |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Pivot Bipolar |
| Version Model Number | 133-2858 |
| Company DUNS | 876542390 |
| Company Name | Ortho Development Corporation |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com |
Device Dimensions
| Lumen/Inner Diameter | 28 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Outer Diameter | 58 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Outer Diameter | 58 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Outer Diameter | 58 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Outer Diameter | 58 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Outer Diameter | 58 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Outer Diameter | 58 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Outer Diameter | 58 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Outer Diameter | 58 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Outer Diameter | 58 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Outer Diameter | 58 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Outer Diameter | 58 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Outer Diameter | 58 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
| Outer Diameter | 58 Millimeter |
| Lumen/Inner Diameter | 28 Millimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00822409015429 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K050966
FDA Product Code
| KWY | Prosthesis, hip, hemi-, femoral, metal/polymer, cemented or uncemented |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2015-08-31 |
On-Brand Devices [Pivot Bipolar]
| 00822409015443 | 28x60mm Bipolar |
| 00822409015436 | 28x59mm Bipolar |
| 00822409015429 | 28x58mm Bipolar |
| 00822409015412 | 28x57mm Bipolar |
| 00822409015405 | 28x56mm Bipolar |
| 00822409015399 | 28x55mm Bipolar |
| 00822409015382 | 28x54mm Bipolar |
| 00822409015375 | 28x53mm Bipolar |
| 00822409015368 | 28x52mm Bipolar |
| 00822409015351 | 28x51mm Bipolar |
| 00822409015344 | 28x50mm Bipolar |
| 00822409015337 | 28x49mm Bipolar |
| 00822409015320 | 28x48mm Bipolar |
| 00822409015313 | 28x47mm Bipolar |
| 00822409015306 | 28x46mm Bipolar |
| 00822409015290 | 22x60mm Bipolar |
| 00822409015283 | 22x59mm Bipolar |
| 00822409015276 | 22x58mm Bipolar |
| 00822409015269 | 22x57mm Bipolar |
| 00822409015252 | 22x56mm Bipolar |
| 00822409015245 | 22x55mm Bipolar |
| 00822409015238 | 22x54mm Bipolar |
| 00822409015221 | 22x53mm Bipolar |
| 00822409015214 | 22x52mm Bipolar |
| 00822409015207 | 22x51mm Bipolar |
| 00822409015191 | 22x50mm Bipolar |
| 00822409015184 | 22x49mm Bipolar |
| 00822409015177 | 22x48mm Bipolar |
| 00822409015160 | 22x47mm Bipolar |
| 00822409015153 | 22x46mm Bipolar |
| 00822409015146 | 22x45mm Bipolar |
| 00822409015139 | 22x44mm Bipolar |
| 00822409015122 | 22x43mm Bipolar |
| 00822409015115 | 22x42mm Bipolar |
| 00822409015108 | 22x41mm Bipolar |
| 00822409015092 | 22x39mm Bipolar |
| 00822409015085 | 22x38mm Bipolar |
| 00822409010226 | 22x40mm Bipolar |
Trademark Results [Pivot Bipolar]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
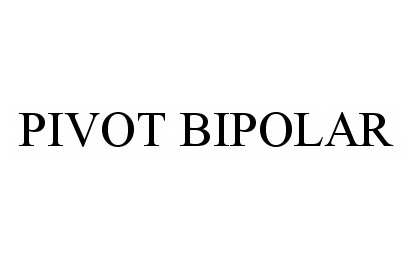 PIVOT BIPOLAR 78594846 3294843 Live/Registered |
Ortho Development Corporation 2005-03-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.