FuseBox LS-FSP-100
GUDID 00840253104267
Endoscopic Video Processor Unit
ENDOCHOICE, INC.
Endoscopic video image processing unit| Primary Device ID | 00840253104267 |
| NIH Device Record Key | 10397011-fbbd-40cf-bac5-c70cae3650d2 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | FuseBox |
| Version Model Number | LS-FSP-100 |
| Catalog Number | LS-FSP-100 |
| Company DUNS | 014154279 |
| Company Name | ENDOCHOICE, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00840253104267 [Primary] |
FDA Product Code
| FDS | Gastroscope And Accessories, Flexible/Rigid |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2019-02-21 |
| Device Publish Date | 2017-08-11 |
On-Brand Devices [FuseBox]
| 00840253114327 | Endoscopic Video Processor Unit |
| 00840253114310 | Endoscopic Processor Unit |
| 00840253104274 | Endoscopic Video Processor Unit |
| 00840253104267 | Endoscopic Video Processor Unit |
| 00840253103932 | Endoscopic video image processor |
Trademark Results [FuseBox]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
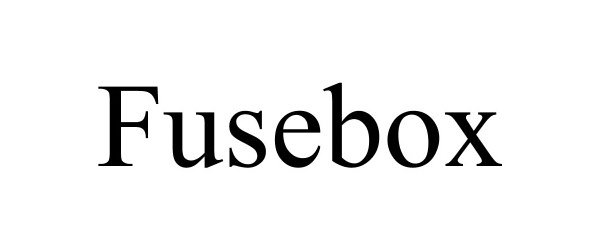 FUSEBOX 90729714 not registered Live/Pending |
FUSEBOX SKINCARE LLC 2021-05-24 |
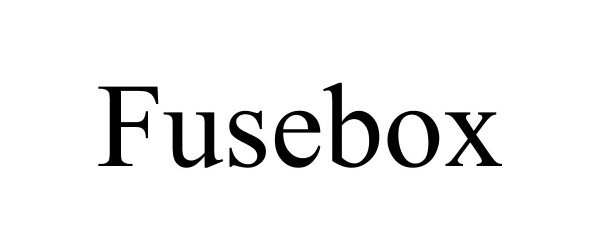 FUSEBOX 90729652 not registered Live/Pending |
FUSEBOX SKINCARE LLC 2021-05-24 |
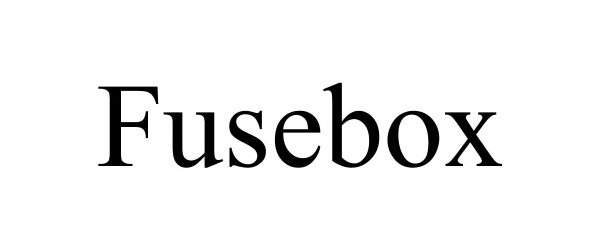 FUSEBOX 90729648 not registered Live/Pending |
FUSEBOX SKINCARE LLC 2021-05-24 |
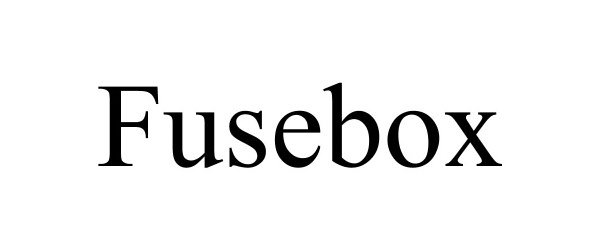 FUSEBOX 90729645 not registered Live/Pending |
FUSEBOX SKINCARE LLC 2021-05-24 |
 FUSEBOX 90313337 not registered Live/Pending |
Mainbridge Health Partners 2020-11-11 |
 FUSEBOX 88839582 not registered Live/Pending |
SPI Labs, LLC 2020-03-18 |
 FUSEBOX 87577141 5471488 Live/Registered |
Providence Medical Technology, Inc. 2017-08-21 |
 FUSEBOX 87320665 not registered Live/Pending |
Essentium Materials, LLC 2017-02-01 |
 FUSEBOX 86880080 5031173 Live/Registered |
Fuse VPN LLC 2016-01-19 |
 FUSEBOX 86550850 5091882 Live/Registered |
Fusebox, LLC 2015-03-02 |
 FUSEBOX 86377112 4952137 Live/Registered |
E-filliate, Inc. 2014-08-26 |
 FUSEBOX 86226767 4620971 Live/Registered |
Endochoice, Inc. 2014-03-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.