SuperClear 3814-875-000-9
GUDID 00840321511188
SuperClear® 10mL Specimen Collection and Transport Tubes, Bulk, Sterile, Tubes Only
Labcon
General specimen receptacle transport container| Primary Device ID | 00840321511188 |
| NIH Device Record Key | 89fe914b-0db3-4a35-8d3e-8cfe24eb6bc4 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | SuperClear |
| Version Model Number | 3814-875-000-9 |
| Catalog Number | 3814-875-000-9 |
| Company DUNS | 029358231 |
| Company Name | Labcon |
| Device Count | 500 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00840321509437 [Primary] |
| GS1 | 00840321511188 [Unit of Use] |
FDA Product Code
| KDT | Container, Specimen Mailer And Storage, Sterile |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-08-09 |
| Device Publish Date | 2023-08-01 |
On-Brand Devices [SuperClear]
| 00840321511171 | SuperClear® 5mL Specimen Collection and Transport Tubes, Bulk, Sterile, Tubes Only |
| 00840321511188 | SuperClear® 10mL Specimen Collection and Transport Tubes, Bulk, Sterile, Tubes Only |
Trademark Results [SuperClear]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SUPERCLEAR 88959688 not registered Live/Pending |
Fiberglass Coatings, Inc. 2020-06-11 |
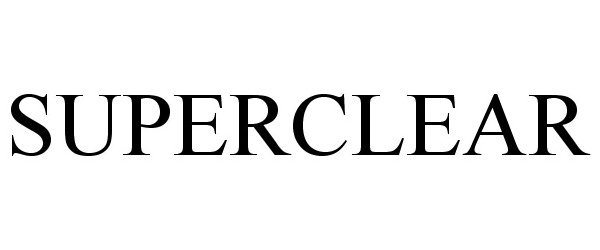 SUPERCLEAR 88464773 not registered Live/Pending |
Global Green World, LLC 2019-06-07 |
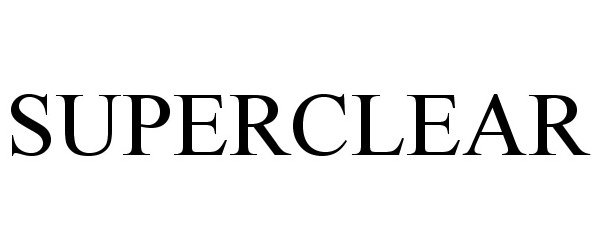 SUPERCLEAR 88171554 not registered Live/Pending |
O'KEEFFE'S, INC. 2018-10-26 |
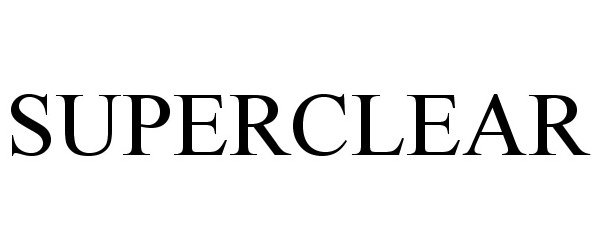 SUPERCLEAR 87903759 not registered Dead/Abandoned |
SUPERCLEAR, LLC 2018-05-02 |
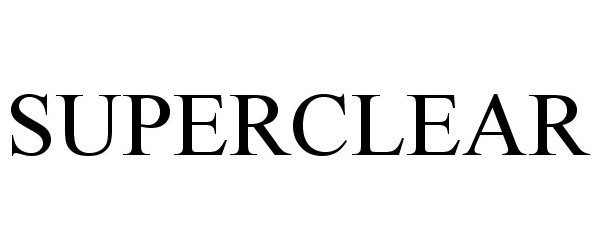 SUPERCLEAR 87903727 not registered Live/Pending |
SUPERCLEAR, LLC 2018-05-02 |
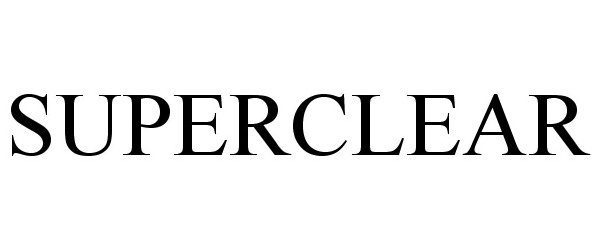 SUPERCLEAR 87903722 not registered Dead/Abandoned |
SUPERCLEAR, LLC 2018-05-02 |
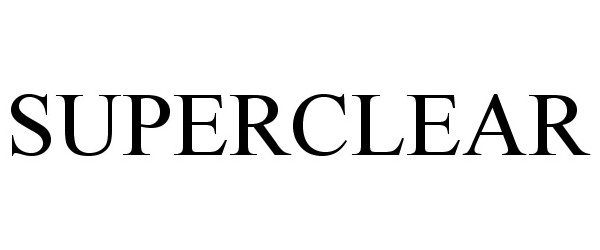 SUPERCLEAR 87903712 not registered Dead/Abandoned |
SUPERCLEAR, LLC 2018-05-02 |
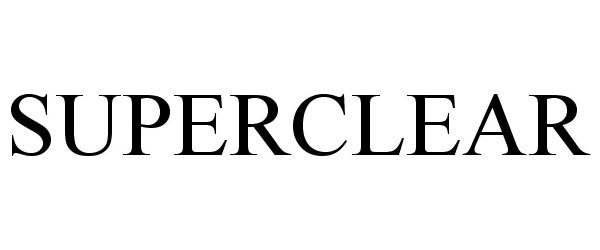 SUPERCLEAR 87903691 not registered Dead/Abandoned |
SUPERCLEAR, LLC 2018-05-02 |
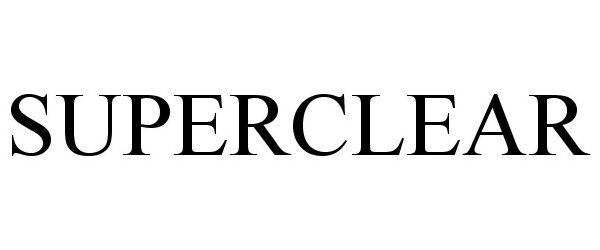 SUPERCLEAR 87882045 not registered Dead/Abandoned |
SUPERCLEAR, LLC 2018-04-18 |
 SUPERCLEAR 87882033 not registered Dead/Abandoned |
SUPERCLEAR, LLC 2018-04-18 |
 SUPERCLEAR 87882022 not registered Dead/Abandoned |
SUPERCLEAR, LLC 2018-04-18 |
 SUPERCLEAR 87882009 not registered Dead/Abandoned |
SUPERCLEAR, LLC 2018-04-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.