Aespire
GUDID 00840682102261
DATEX-OHMEDA INC
Anaesthesia workstation, general-purpose| Primary Device ID | 00840682102261 |
| NIH Device Record Key | 07c049e5-e2d6-472b-80e9-d5d2056c59e7 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Aespire |
| Version Model Number | 7900 |
| Company DUNS | 129501685 |
| Company Name | DATEX-OHMEDA INC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Unsafe |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Operating and Storage Conditions
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Atmospheric Pressure | Between 49.99 KiloPascal and 106.66 KiloPascal |
| Storage Environment Temperature | Between -25 Degrees Celsius and 65 Degrees Celsius |
| Storage Environment Humidity | Between 10 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00840682102261 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K050626
FDA Product Code
| BSZ | Gas-machine, anesthesia |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2015-09-23 |
On-Brand Devices [Aespire]
| 00840682102285 | View |
| 00840682102261 | 7900 |
Trademark Results [Aespire]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AESPIRE 88362915 5879323 Live/Registered |
Aespire 2019-03-29 |
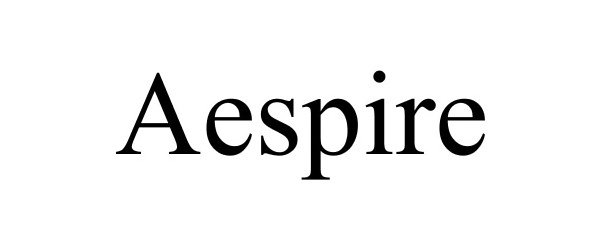 AESPIRE 85503773 4190245 Dead/Cancelled |
Brian Sooy & Co. 2011-12-26 |
 AESPIRE 77886069 not registered Dead/Abandoned |
Cyberonics, Inc. 2009-12-04 |
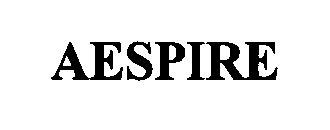 AESPIRE 76362579 2976841 Live/Registered |
DATEX-OHMEDA, INC. 2002-01-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.