5008X
GUDID 00840861102884
5008X + CDX
Fresenius Medical Care AG
Haemodialysis system, institutional| Primary Device ID | 00840861102884 |
| NIH Device Record Key | d96fbba9-dc87-4144-9b56-5f7dbd49408b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | 5008X |
| Version Model Number | M204431 |
| Company DUNS | 324661834 |
| Company Name | Fresenius Medical Care AG |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Unsafe |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx | |
| Phone | 1 (800) 227-2572 |
| xx@xx.xx |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00840861102884 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K243505
FDA Product Code
| KDI | Dialyzer, High Permeability With Or Without Sealed Dialysate System |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2025-08-11 |
| Device Publish Date | 2025-08-01 |
On-Brand Devices [5008X]
| 00840861102884 | 5008X + CDX |
| 00840861102877 | 5008X + CLiC |
| 00840861102860 | 5008X Standard |
Trademark Results [5008X]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
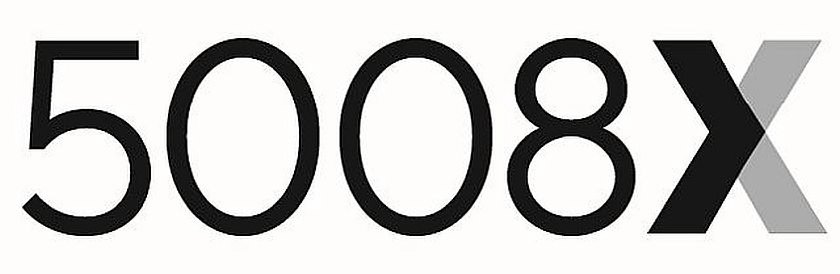 5008X 90752280 not registered Live/Pending |
Fresenius Medical Care Holdings, Inc. 2021-06-03 |
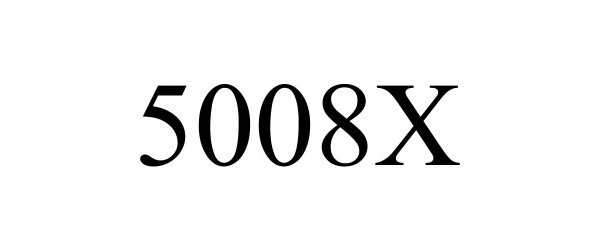 5008X 90746981 not registered Live/Pending |
Fresenius Medical Care Holdings, Inc. 2021-06-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.