RAVEN
GUDID 00840996184083
RAVEN,5.0X55
Choice Spine, LP
Bone-screw internal spinal fixation system, non-sterile| Primary Device ID | 00840996184083 |
| NIH Device Record Key | 1d13f9e7-b041-47a4-8b95-640bbefd0161 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | RAVEN |
| Version Model Number | YT35-5055 |
| Company DUNS | 078293017 |
| Company Name | Choice Spine, LP |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net | |
| Phone | +1(865)246-3333 |
| info@choicespine.net |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00840996184083 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K191367
FDA Product Code
| MAX | Intervertebral fusion device with bone graft, lumbar |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
[00840996184083]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-02-13 |
| Device Publish Date | 2023-02-03 |
On-Brand Devices [RAVEN]
| 00840996184137 | RAVEN,5.5X60 |
| 00840996184120 | RAVEN,5.5X55 |
| 00840996184113 | RAVEN,5.5X45 |
| 00840996184106 | RAVEN,5.5X50 |
| 00840996184090 | RAVEN,5.0X60 |
| 00840996184083 | RAVEN,5.0X55 |
| 00840996184076 | RAVEN,5.0X50 |
| 00840996184069 | RAVEN,5.0X45 |
Trademark Results [RAVEN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
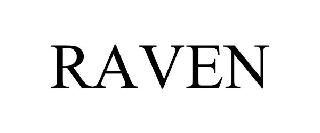 RAVEN 98537473 not registered Live/Pending |
California Exotic Novelties, LLC 2024-05-07 |
 RAVEN 98507073 not registered Live/Pending |
Raven Windows Inc. 2024-04-18 |
 RAVEN 98501913 not registered Live/Pending |
RA Capital Management, LLC 2024-04-16 |
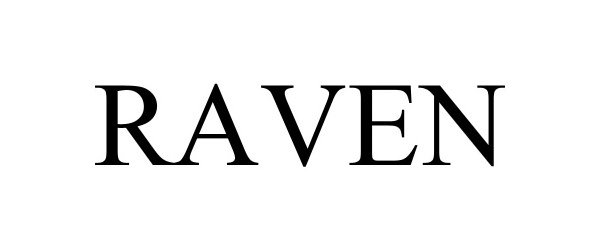 RAVEN 98501912 not registered Live/Pending |
RA Capital Management, LLC 2024-04-16 |
 RAVEN 98401467 not registered Live/Pending |
Raven Industries, Inc. 2024-02-12 |
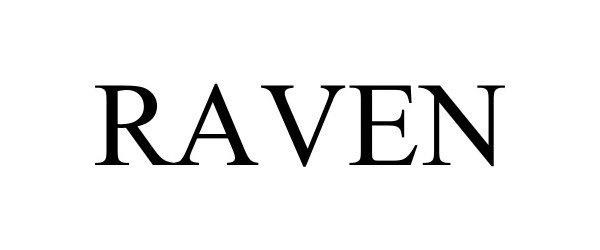 RAVEN 98260363 not registered Live/Pending |
Rajant Corporation 2023-11-08 |
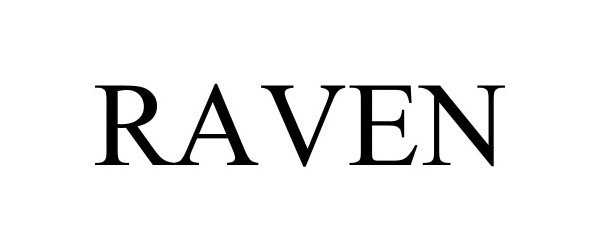 RAVEN 97886299 not registered Live/Pending |
Alliance Machine Systems International,LLC 2023-04-13 |
 RAVEN 97861796 not registered Live/Pending |
Wei, Xiao 2023-03-28 |
 RAVEN 97861795 not registered Live/Pending |
Wei, Xiao 2023-03-28 |
 RAVEN 97861496 not registered Live/Pending |
Wei, Xiao 2023-03-28 |
 RAVEN 97702459 not registered Live/Pending |
Raven Custom Homes, Inc. 2022-12-02 |
 RAVEN 97318326 not registered Live/Pending |
Eagle Eye LLC 2022-03-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.