REEL LEGENDS
GUDID 00841543135534
SUNGLASS
Inspecs U.S.A., L.C.
Spectacle frame| Primary Device ID | 00841543135534 |
| NIH Device Record Key | d29d9783-802d-43a9-83b5-64724721234c |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | REEL LEGENDS |
| Version Model Number | BRS-SAMARA-104P |
| Company DUNS | 845937478 |
| Company Name | Inspecs U.S.A., L.C. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00841543135534 [Primary] |
FDA Product Code
| HQY | Sunglasses (non-prescription including photosensitive) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-12-09 |
| Device Publish Date | 2022-12-01 |
On-Brand Devices [REEL LEGENDS]
| 00841543135589 | SUNGLASS |
| 00841543135565 | SUNGLASS |
| 00841543135534 | SUNGLASS |
Trademark Results [REEL LEGENDS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REEL LEGENDS 98696704 not registered Live/Pending |
BDSRCO, Inc. 2024-08-13 |
 REEL LEGENDS 88596813 not registered Live/Pending |
BDSRCO, Inc. 2019-08-28 |
 REEL LEGENDS 87440849 5439648 Live/Registered |
BDSRCO, Inc. 2017-05-08 |
 REEL LEGENDS 86724058 not registered Dead/Abandoned |
BDSRCO, Inc. 2015-08-13 |
 REEL LEGENDS 86226899 4827936 Live/Registered |
BDSRCO, Inc. 2014-03-20 |
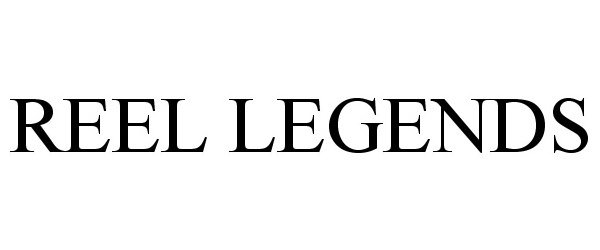 REEL LEGENDS 85982845 4765041 Live/Registered |
BDSRCO, Inc. 2013-04-29 |
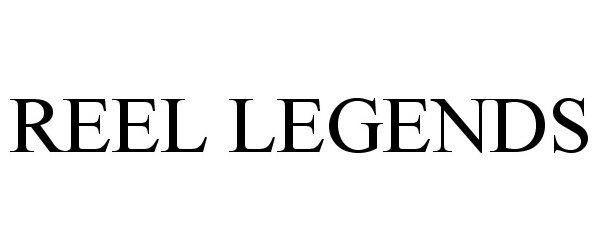 REEL LEGENDS 85981039 4508204 Live/Registered |
BDSRCO, Inc. 2013-04-29 |
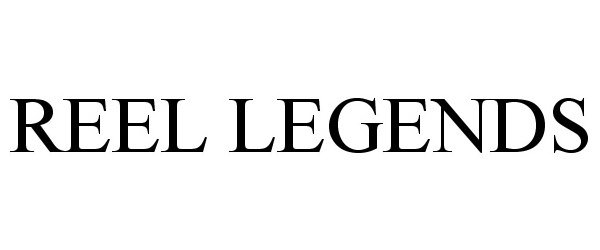 REEL LEGENDS 85917897 not registered Dead/Abandoned |
BDSRCO, Inc. 2013-04-29 |
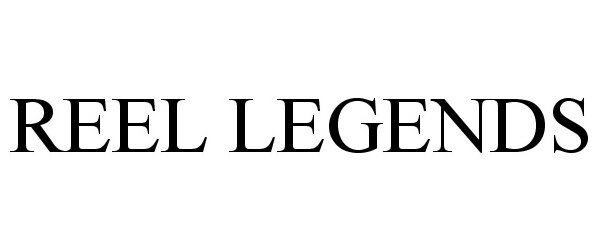 REEL LEGENDS 85891790 4544782 Live/Registered |
BDSRCO, Inc. 2013-04-01 |
 REEL LEGENDS 85276119 4155357 Live/Registered |
BDSRCO, INC. 2011-03-24 |
 REEL LEGENDS 78446228 3024776 Dead/Cancelled |
WMS GAMING INC. 2004-07-06 |
 REEL LEGENDS 76317553 2755158 Live/Registered |
BDSRCO 2001-09-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.