Simplify® Cervical Artificial Disc DG-SM
GUDID 00843285100931
Simplify® Depth Gauge, Size SM
Simplify Medical, Inc.
Orthopaedic implant/instrument depth limiter| Primary Device ID | 00843285100931 |
| NIH Device Record Key | d538276f-22e0-4d0a-8237-5a8f92284c9d |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Simplify® Cervical Artificial Disc |
| Version Model Number | F |
| Catalog Number | DG-SM |
| Company DUNS | 075663686 |
| Company Name | Simplify Medical, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | 650-947-3472 |
| info@simplifymedical.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00843285100931 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Approval: P200022
FDA Product Code
| MJO | Prosthesis, Intervertebral Disc |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
[00843285100931]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-12-22 |
| Device Publish Date | 2020-12-14 |
On-Brand Devices [Simplify® Cervical Artificial Disc ]
| 00843285101754 | Simplify® Inserter, Size LG |
| 00843285101747 | Simplify® Inserter, Size MD |
| 00843285101730 | Simplify® Inserter, Size SM |
| 00843285101693 | Simplify® Slot Cutter, Size MD, 4mm |
| 00843285101686 | Simplify® Slot Cutter, Size SM, 4mm |
| 00843285100955 | Simplify® Depth Gauge, Size LG |
| 00843285100948 | Simplify® Depth Gauge, Size MD |
| 00843285100931 | Simplify® Depth Gauge, Size SM |
| 00843285100771 | Simplify® Trial, Size LG, Height 6, 5° Lordosis |
| 00843285100764 | Simplify® Trial, Size LG, Height 6 |
| 00843285100757 | Simplify® Trial, Size LG, Height 5, 5° Lordosis |
| 00843285100740 | Simplify® Trial, Size LG, Height 5 |
| 00843285100733 | Simplify® Trial, Size MD, Height 6, 5° Lordosis |
| 00843285100726 | Simplify® Trial, Size MD, Height 6 |
| 00843285100719 | Simplify® Trial, Size MD, Height 5, 5° Lordosis |
| 00843285100702 | Simplify® Trial, Size MD, Height 5 |
| 00843285100696 | Simplify® Trial, Size MD, Height 4 |
| 00843285100689 | Simplify® Trial, Size SM, Height 6 |
| 00843285100665 | Simplify® Trial, Size SM, Height 5 |
| 00843285100627 | Simplify® Trial, Size SM, Height 4 |
| 00843285100139 | Simplify® Cervical Artificial Disc Size LG, Height 6, 5° Lordosis |
| 00843285100122 | Simplify® Cervical Artificial Disc Size LG, Height 6 |
| 00843285100115 | Simplify® Cervical Artificial Disc Size LG, Height 5, 5° Lordosis |
| 00843285100108 | Simplify® Cervical Artificial Disc Size LG, Height 5 |
| 00843285100092 | Simplify® Cervical Artificial Disc Size MD, Height 6, 5° Lordosis |
| 00843285100085 | Simplify® Cervical Artificial Disc Size MD, Height 6 |
| 00843285100078 | Simplify® Cervical Artificial Disc Size MD, Height 5, 5° Lordosis |
| 00843285100061 | Simplify® Cervical Artificial Disc Size MD, Height 5 |
| 00843285100054 | Simplify® Cervical Artificial Disc Size MD, Height 4 |
| 00843285100047 | Simplify® Cervical Artificial Disc Size SM, Height 6 |
| 00843285100030 | Simplify® Cervical Artificial Disc Size SM, Height 5, 5° Lordosis |
| 00843285100023 | Simplify® Cervical Artificial Disc Size SM, Height 5 |
| 00843285100016 | Simplify® Cervical Artificial Disc Size SM, Height 4, 5° Lordosis |
| 00843285100009 | Simplify® Cervical Artificial Disc Size SM, Height 4 |
| 00843285101709 | Simplify® Slot Cutter, Size SM, 5/6mm |
Trademark Results [Simplify]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
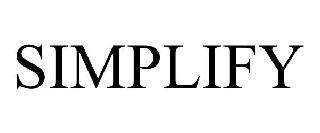 SIMPLIFY 98596744 not registered Live/Pending |
Simplify Workforce, Inc 2024-06-12 |
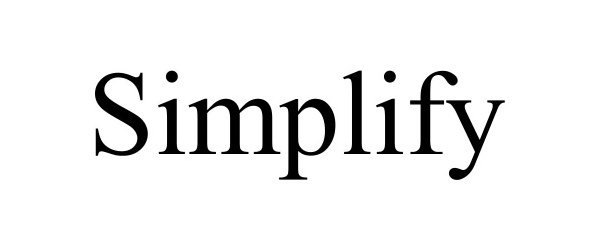 SIMPLIFY 98239372 not registered Live/Pending |
SIMPLIFYAI LLC 2023-10-25 |
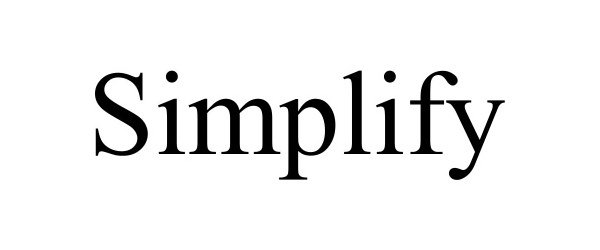 SIMPLIFY 98102133 not registered Live/Pending |
SIMPLIFYAI LLC 2023-07-26 |
 SIMPLIFY 90156004 not registered Live/Pending |
International Wholesale Inc 2020-09-03 |
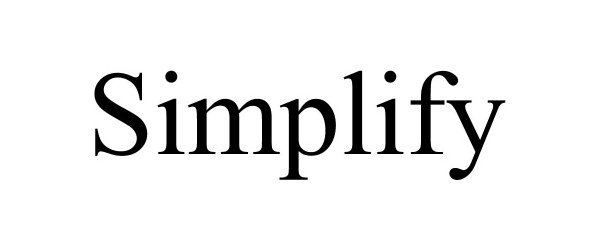 SIMPLIFY 88838510 not registered Live/Pending |
Organizingbean LLC 2020-03-18 |
 SIMPLIFY 87924387 not registered Live/Pending |
Lee, Benjamin L. 2018-05-16 |
 SIMPLIFY 87779370 not registered Dead/Abandoned |
Welspun India Limited 2018-02-01 |
 SIMPLIFY 87653913 not registered Dead/Abandoned |
Name Rite, L.L.C. 2017-10-20 |
 SIMPLIFY 87653900 not registered Dead/Abandoned |
Name Rite, L.L.C. 2017-10-20 |
 SIMPLIFY 87653894 not registered Dead/Abandoned |
Name Rite, L.L.C. 2017-10-20 |
 SIMPLIFY 87653888 not registered Dead/Abandoned |
Name Rite, L.L.C. 2017-10-20 |
 SIMPLIFY 87653881 not registered Dead/Abandoned |
Name Rite, L.L.C. 2017-10-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.