D-10 2200149
GUDID 00847817003417
D-10 Sample Vials
BIO-RAD LABORATORIES, INC.
Haemoglobin A2 (HbA2) IVD, reagent| Primary Device ID | 00847817003417 |
| NIH Device Record Key | 1221063d-fc1f-4907-b009-65a55e7dc886 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | D-10 |
| Version Model Number | 220-0149 |
| Catalog Number | 2200149 |
| Company DUNS | 132528295 |
| Company Name | BIO-RAD LABORATORIES, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)224-6723 |
| diagcs@bio-rad.com | |
| Phone | +1(800)224-6723 |
| diagcs@bio-rad.com | |
| Phone | +1(800)224-6723 |
| diagcs@bio-rad.com | |
| Phone | +1(800)224-6723 |
| diagcs@bio-rad.com | |
| Phone | +1(800)224-6723 |
| diagcs@bio-rad.com | |
| Phone | +1(800)224-6723 |
| diagcs@bio-rad.com | |
| Phone | +1(800)224-6723 |
| diagcs@bio-rad.com | |
| Phone | +1(800)224-6723 |
| diagcs@bio-rad.com |
Operating and Storage Conditions
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00847817003417 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K031043
FDA Product Code
| LCP | ASSAY, GLYCOSYLATED HEMOGLOBIN |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 6 |
| Public Version Date | 2020-01-23 |
| Device Publish Date | 2016-09-12 |
On-Brand Devices [D-10]
| 00847817003462 | D-10 Analytical Cartridge Hemoglobin A1c Program or Hemoglobin A2/F/A1c Program |
| 00847817003417 | D-10 Sample Vials |
| 00847817003356 | D-10 Hemoglobin A1c Program Calibrator/Diluent Set |
| 00847817003318 | D-10 Analytical Cartridge Hemoglobin A1c Program |
| 00847817003301 | D-10 Wash/Diluent Solution |
| 00847817003295 | D-10 Hemoglobin A1c Program Elution Buffer 2 |
| 00847817003288 | D-10 Hemoglobin A1c Program Elution Buffer 1 |
| 00847817003271 | D-10 Hemoglobin A1c Supplemental Reagent Pack |
| 00847817000089 | D-10 Hemoglobin A1c Program REORDER PACK |
| 03610521164319 | D-10 Hemoglobin A1c Program Calibrator/Diluent Set |
| 03610521164302 | D-10 Hemoglobin A1c Program Analytical Cartridge |
| 03610520530108 | D-10 Hemoglobin A1c Program |
| 03610522126750 | D-10 Hemoglobin Testing System |
Trademark Results [D-10]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
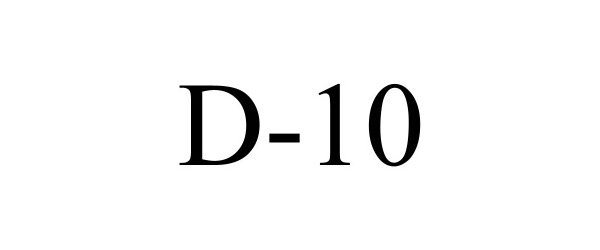 D-10 97820230 not registered Live/Pending |
Bio-Rad Laboratories, Inc. 2023-03-02 |
 D-10 74502892 1884589 Dead/Cancelled |
Medeco Security Locks, Inc. 1994-03-21 |
 D-10 73666338 1475627 Dead/Cancelled |
MEDECO SECURITY LOCKS, INC. 1987-06-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.