BioPlex® 2200 665-3465A
GUDID 00847865001281
U.S. IFU Manual and CDs, BioPlex 2200 HIV Ag-Ab IFU 665-3465A
BIO-RAD LABORATORIES, INC.
Laboratory instrument/analyser application software IVD| Primary Device ID | 00847865001281 |
| NIH Device Record Key | 2027283b-ac39-4640-829c-7191868bbc1a |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | BioPlex® 2200 |
| Version Model Number | SW4.2_v4 |
| Catalog Number | 665-3465A |
| Company DUNS | 884513334 |
| Company Name | BIO-RAD LABORATORIES, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 1-800-224-6723 |
| TechSupport.USSD@bio-rad.com | |
| Phone | 1-800-224-6723 |
| TechSupport.USSD@bio-rad.com | |
| Phone | 1-800-224-6723 |
| TechSupport.USSD@bio-rad.com | |
| Phone | 1-800-224-6723 |
| TechSupport.USSD@bio-rad.com | |
| Phone | 1-800-224-6723 |
| TechSupport.USSD@bio-rad.com | |
| Phone | 1-800-224-6723 |
| TechSupport.USSD@bio-rad.com | |
| Phone | 1-800-224-6723 |
| TechSupport.USSD@bio-rad.com | |
| Phone | 1-800-224-6723 |
| TechSupport.USSD@bio-rad.com | |
| Phone | 1-800-224-6723 |
| TechSupport.USSD@bio-rad.com | |
| Phone | 1-800-224-6723 |
| TechSupport.USSD@bio-rad.com | |
| Phone | 1-800-224-6723 |
| TechSupport.USSD@bio-rad.com | |
| Phone | 1-800-224-6723 |
| TechSupport.USSD@bio-rad.com | |
| Phone | 1-800-224-6723 |
| TechSupport.USSD@bio-rad.com | |
| Phone | 1-800-224-6723 |
| TechSupport.USSD@bio-rad.com |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00847865001281 [Primary] |
FDA Product Code
| MZF | Test, Hiv Detection |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2018-03-29 |
| Device Publish Date | 2015-08-28 |
On-Brand Devices [BioPlex® 2200 ]
| 00847865019255 | BioPlex 2200 HIV Ag-Ab Reagent Pack |
| 00847865018487 | BioPlex 2200, HIV Ag-Ab Control Lot Data |
| 00847865018470 | BioPlex 2200, HIV Ag-Ab Calibrator Lot Data |
| 00847865011105 | BioPlex® 2200 Manual Reagent Pack Piercer |
| 00847865001281 | U.S. IFU Manual and CDs, BioPlex 2200 HIV Ag-Ab IFU 665-3465A |
| 03610520506493 | BioPlex 2200, HIV Ag-Ab Control Set |
| 03610520506486 | BioPlex 2200 HIV Ag-Ab Calibrator Set |
| 03610520519813 | BioPlex® 2200 HIV Ag-Ab Assay Protocol Files CD SW4.2_v4 |
Trademark Results [BioPlex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
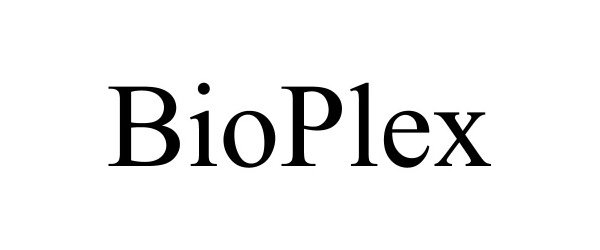 BIOPLEX 90741204 not registered Live/Pending |
JAJAS IP HOLDINGS LLC 2021-05-28 |
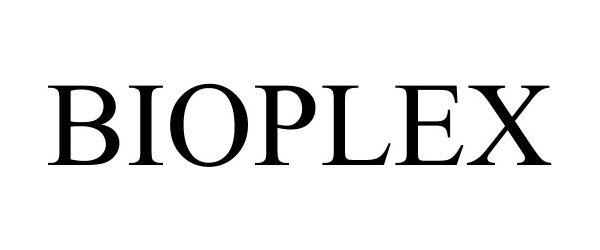 BIOPLEX 90689684 not registered Live/Pending |
Chevron Phillips Chemical Company LP 2021-05-04 |
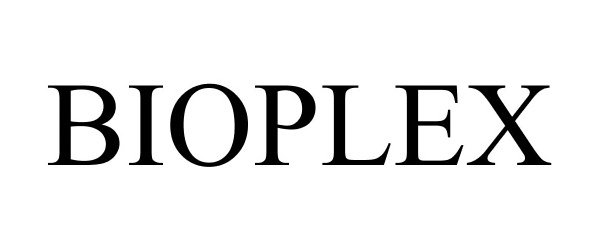 BIOPLEX 87591383 not registered Dead/Abandoned |
LIVING BLOOM LLC 2017-08-31 |
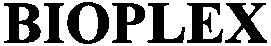 BIOPLEX 79002110 not registered Dead/Abandoned |
Avaris AB 2004-03-29 |
 BIOPLEX 78608479 not registered Dead/Abandoned |
COSMOCEL S.A. 2005-04-14 |
 BIOPLEX 78187751 not registered Dead/Abandoned |
Interpore Orthopaedics, Inc 2002-11-21 |
 BIOPLEX 76497977 2808329 Live/Registered |
Biosil Technologies Inc. 2003-03-11 |
 BIOPLEX 76381236 2763512 Live/Registered |
Alltech, Inc. 2002-03-12 |
 BIOPLEX 75112637 not registered Dead/Abandoned |
Personal Care Group, Inc. 1996-06-03 |
 BIOPLEX 74261470 not registered Dead/Abandoned |
STERLING WINTHROP INC. 1992-04-01 |
 BIOPLEX 73595407 1514277 Dead/Cancelled |
DATASCOPE CORP 1986-04-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.