Signage
GUDID 00848530100032
Oxygen No Smoking Signs - 50/pack
SUNSET HEALTHCARE SOLUTIONS, INC.
Oxygen administration kit| Primary Device ID | 00848530100032 |
| NIH Device Record Key | 3ea079a2-eedf-485d-948d-d0ca685a3f08 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Signage |
| Version Model Number | TAGNSSIGN |
| Company DUNS | 141668116 |
| Company Name | SUNSET HEALTHCARE SOLUTIONS, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 8775786738 |
| customerservice@sunsethcs.com | |
| Phone | 8775786738 |
| customerservice@sunsethcs.com | |
| Phone | 8775786738 |
| customerservice@sunsethcs.com | |
| Phone | 8775786738 |
| customerservice@sunsethcs.com | |
| Phone | 8775786738 |
| customerservice@sunsethcs.com | |
| Phone | 8775786738 |
| customerservice@sunsethcs.com | |
| Phone | 8775786738 |
| customerservice@sunsethcs.com | |
| Phone | 8775786738 |
| customerservice@sunsethcs.com | |
| Phone | 8775786738 |
| customerservice@sunsethcs.com | |
| Phone | 8775786738 |
| customerservice@sunsethcs.com | |
| Phone | 8775786738 |
| customerservice@sunsethcs.com | |
| Phone | 8775786738 |
| customerservice@sunsethcs.com | |
| Phone | 8775786738 |
| customerservice@sunsethcs.com | |
| Phone | 8775786738 |
| customerservice@sunsethcs.com | |
| Phone | 8775786738 |
| customerservice@sunsethcs.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00848530100032 [Primary] |
FDA Product Code
| OGL | Oxygen Administration Kit |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2025-06-11 |
| Device Publish Date | 2025-06-03 |
Devices Manufactured by SUNSET HEALTHCARE SOLUTIONS, INC.
| 00848530108168 - Regulators | 2026-02-10 Private Labeled - Standard CGA 870 Regulator, 1-DISS, 0-25LPM |
| 10848530108172 - CPAP Accessory | 2026-02-10 Sunset Nasal Gel Pad - 5/PK |
| 00848530108267 - Filter - Vent | 2026-02-10 Luisa Gross Particle Internal Filter - 1/PK |
| 10848530108271 - CPAP Tubing | 2026-02-10 Adapter for Resmed S10 Heated CPAP Tube System |
| 00848530108298 - CPAP Filter | 2026-02-04 Filter, USA, Standard S9, AirSense 10, AirCurve, 2/pack |
| 00848530107932 - Nebulizer and Compressor | 2026-02-03 Sunset Compressor Nebulizer w/ Disposable Adult Neb Kit |
| 10848530108127 - Regulator | 2026-02-03 Standard CGA 870 Regulator, 1-DISS, 0-25LPM |
| 00848530108137 - CPAP Filter | 2026-02-03 Filter, USA, Airsense 11, Standard, 50/Pack |
Trademark Results [Signage]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
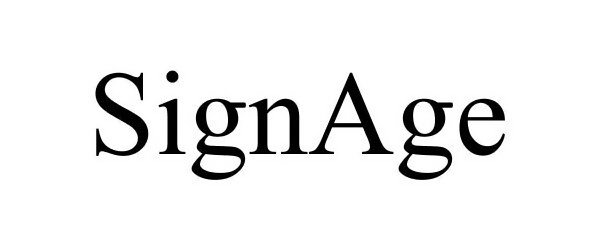 SIGNAGE 86153130 not registered Dead/Abandoned |
SAMSUNG ELECTRONICS CO., LTD. 2013-12-27 |
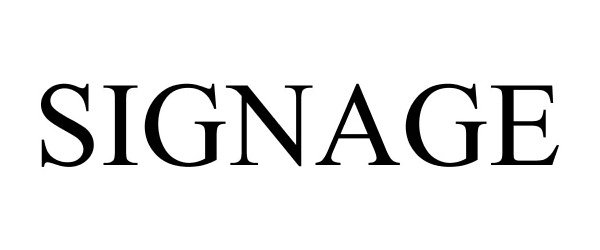 SIGNAGE 77497459 not registered Dead/Abandoned |
Parker, Delando S 2008-06-12 |
 SIGNAGE 74180182 1741240 Dead/Cancelled |
FARMER, KENNETH R. 1991-06-27 |
 SIGNAGE 73676618 1493206 Dead/Cancelled |
ROBERT BRUCE INC. 1987-08-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.