DoseRite DR0084NL-12
GUDID 00850000610064
DoseRite Anal Fissure Pack, Metric
SURGIN SURGICAL INSTRUMENTATION, INC.
Rectal medication applicator, reusable| Primary Device ID | 00850000610064 |
| NIH Device Record Key | da1610a5-15e9-458b-a089-26394643c61a |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | DoseRite |
| Version Model Number | DR0084NL-12 |
| Catalog Number | DR0084NL-12 |
| Company DUNS | 076656164 |
| Company Name | SURGIN SURGICAL INSTRUMENTATION, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | false |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00850000610064 [Primary] |
FDA Product Code
| KYZ | Syringe, Irrigating (Non Dental) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-07-18 |
| Device Publish Date | 2023-07-10 |
On-Brand Devices [DoseRite]
| 00850000610118 | DoseRite Anal Applicator Pack |
| 00850000610064 | DoseRite Anal Fissure Pack, Metric |
| 00850000610033 | DoseRite Reusable Pack, Metric |
| 00850000610026 | DoseRite Reusable Pack (Case of 10) |
| 00850000610002 | DoseRite Anal Fissure Pack, Reusable, Metric |
Trademark Results [DoseRite]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DOSERITE 97843966 not registered Live/Pending |
Squared Products of Ohio LLC 2023-03-17 |
 DOSERITE 88221981 not registered Live/Pending |
SQUARED PRODUCTS OF OHIO LLC 2018-12-08 |
 DOSERITE 87048163 5538082 Live/Registered |
Surgin Medical, Inc. 2016-05-24 |
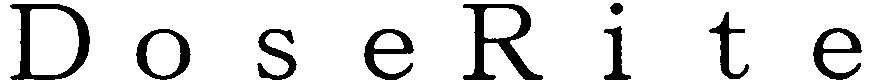 DOSERITE 79142171 4792386 Live/Registered |
Canon Medical Systems Corporation 2013-11-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.