Amethyst MG5803
GUDID 00850005229544
Amethyst Nitrile Exam Gloves, Reflex Blue, Large
Medgluv Inc.
Nitrile examination/treatment glove, non-powdered, non-antimicrobial| Primary Device ID | 00850005229544 |
| NIH Device Record Key | 1124a78e-2c35-4fae-b6d6-919af3c245ab |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Amethyst |
| Version Model Number | MG5803 |
| Catalog Number | MG5803 |
| Company DUNS | 015809044 |
| Company Name | Medgluv Inc. |
| Device Count | 100 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(866)633-4588 |
| sales@medgluv.com | |
| Phone | +1(866)633-4588 |
| sales@medgluv.com | |
| Phone | +1(866)633-4588 |
| sales@medgluv.com | |
| Phone | +1(866)633-4588 |
| sales@medgluv.com | |
| Phone | +1(866)633-4588 |
| sales@medgluv.com | |
| Phone | +1(866)633-4588 |
| sales@medgluv.com | |
| Phone | +1(866)633-4588 |
| sales@medgluv.com | |
| Phone | +1(866)633-4588 |
| sales@medgluv.com | |
| Phone | +1(866)633-4588 |
| sales@medgluv.com | |
| Phone | +1(866)633-4588 |
| sales@medgluv.com | |
| Phone | +1(866)633-4588 |
| sales@medgluv.com | |
| Phone | +1(866)633-4588 |
| sales@medgluv.com | |
| Phone | +1(866)633-4588 |
| sales@medgluv.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00810145951323 [Unit of Use] |
| GS1 | 00850005229506 [Primary] |
| GS1 | 00850005229544 [Package] Contains: 00850005229506 Package: 100/bx, 10bx/cs [10 Units] In Commercial Distribution |
FDA Product Code
| QDO | Fentanyl and other opioid protection glove |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-11-08 |
| Device Publish Date | 2023-10-31 |
On-Brand Devices [Amethyst]
| 00850005229353 | Amethyst Nitrile Exam Gloves, Reflex Blue, Extra Small |
| 00850005229551 | Amethyst Nitrile Exam Gloves, Reflex Blue, Extra Large |
| 00850005229544 | Amethyst Nitrile Exam Gloves, Reflex Blue, Large |
| 00850005229537 | Amethyst Nitrile Exam Gloves, Reflex Blue, Medium |
| 00850005229520 | Amethyst Nitrile Exam Gloves, Reflex Blue, Small |
Trademark Results [Amethyst]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
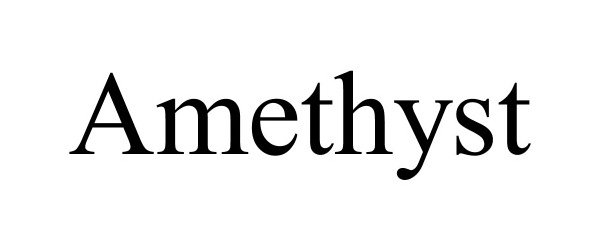 AMETHYST 90733149 not registered Live/Pending |
Xuchang Juyou Hair Products Co., Ltd. 2021-05-25 |
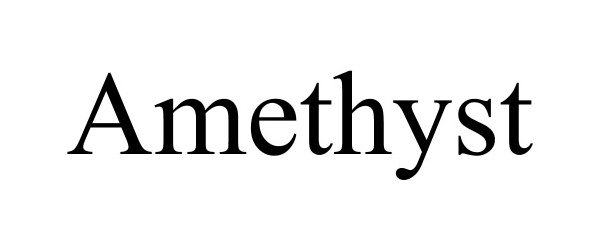 AMETHYST 90208288 not registered Live/Pending |
BOA Biomedical Inc 2020-09-24 |
 AMETHYST 88862081 not registered Live/Pending |
The Sugar Art Inc. FORMERLY ECG Supplies CORPORATION 2020-04-07 |
 AMETHYST 88586698 not registered Live/Pending |
The Boston Consulting Group, Inc. 2019-08-21 |
 AMETHYST 88374083 not registered Live/Pending |
Burnt Church Distillery, LLC 2019-04-05 |
 AMETHYST 87831143 5585496 Live/Registered |
Amethyst Coffee Company, LLC 2018-03-12 |
 AMETHYST 87831131 5585493 Live/Registered |
Amethyst Coffee Company, LLC 2018-03-12 |
 AMETHYST 87335876 5295960 Live/Registered |
Dulcich, Inc. 2017-02-14 |
 AMETHYST 86133736 4567679 Live/Registered |
SHOYEIDO USA, INC. 2013-12-03 |
 AMETHYST 86030439 not registered Dead/Abandoned |
Ellensburg Distillery LLC 2013-08-06 |
 AMETHYST 85216517 4085051 Dead/Cancelled |
MORAN, LI 2011-01-13 |
 AMETHYST 85158619 4168640 Dead/Cancelled |
LI MORAN 2010-10-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.