DAYSPRING F-0040-02
GUDID 00850012532637
LEG GARMENT, DAYSPRING, LONG
Koya Medical, Inc.
Wearable sequential venous compression system| Primary Device ID | 00850012532637 |
| NIH Device Record Key | a39b793f-8f7b-431c-81f5-f1fa606f887a |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | DAYSPRING |
| Version Model Number | F-0040-02 |
| Catalog Number | F-0040-02 |
| Company DUNS | 100673310 |
| Company Name | Koya Medical, Inc. |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(415)209-5035 |
| clee@koyamedical.com | |
| Phone | +1(415)209-5035 |
| clee@koyamedical.com | |
| Phone | +1(415)209-5035 |
| clee@koyamedical.com | |
| Phone | +1(415)209-5035 |
| clee@koyamedical.com | |
| Phone | +1(415)209-5035 |
| clee@koyamedical.com |
Operating and Storage Conditions
| Storage Environment Temperature | Between -20 Degrees Celsius and 60 Degrees Celsius |
| Storage Environment Temperature | Between -20 Degrees Celsius and 60 Degrees Celsius |
| Storage Environment Temperature | Between -20 Degrees Celsius and 60 Degrees Celsius |
| Storage Environment Temperature | Between -20 Degrees Celsius and 60 Degrees Celsius |
| Storage Environment Temperature | Between -20 Degrees Celsius and 60 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00850012532637 [Primary] |
FDA Product Code
| JOW | SLEEVE, LIMB, COMPRESSIBLE |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-05-24 |
| Device Publish Date | 2022-05-16 |
On-Brand Devices [DAYSPRING]
| 00850012532651 | CONTROLLER, DAYSPRING, LITE |
| 00850012532644 | LOWER LEG GARMENT, DAYSPRING |
| 00850012532637 | LEG GARMENT, DAYSPRING, LONG |
| 00850012532620 | LEG GARMENT, DAYSPRING, STANDARD |
| 00850012532613 | Dayspring Mobile Application |
| 00850012532798 | LEG GARMENT, DAYSPRING, LONG, RIGHT |
| 00850012532781 | LEG GARMENT, DAYSPRING, LONG, LEFT |
| 00850012532774 | LEG GARMENT, DAYSPRING, MEDIUM, RIGHT |
| 00850012532767 | LEG GARMENT, DAYSPRING, MEDIUM, LEFT |
| 00850012532750 | LEG GARMENT, DAYSPRING, SHORT, RIGHT |
| 00850012532743 | LEG GARMENT, DAYSPRING, SHORT, LEFT |
| 00850012532736 | LOWER LEG GARMENT |
| 00850012532729 | ARM GARMENT, LONG |
| 00850012532712 | ARM GARMENT, MED |
| 00850012532705 | ARM GARMENT, SHORT |
Trademark Results [DAYSPRING]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
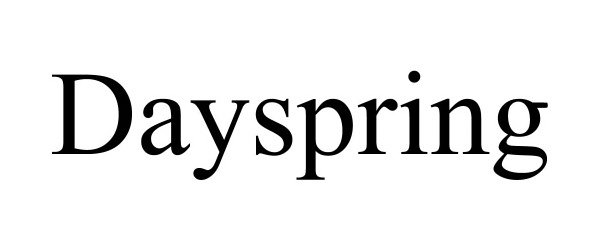 DAYSPRING 90152250 not registered Live/Pending |
FGBC, LLC 2020-09-01 |
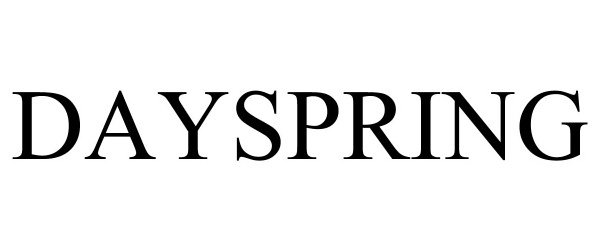 DAYSPRING 88730687 not registered Live/Pending |
KOYA, INC. 2019-12-17 |
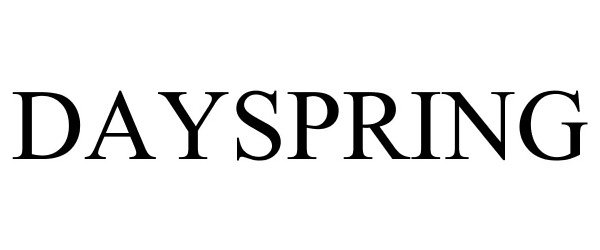 DAYSPRING 87701472 5852321 Live/Registered |
DaySpring Cards, Inc. 2017-11-29 |
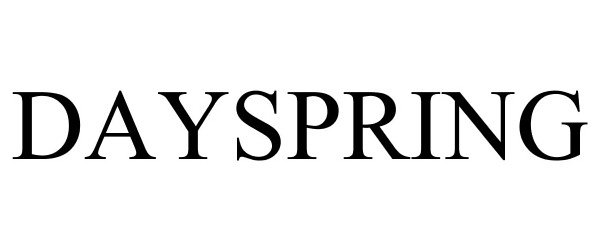 DAYSPRING 87603735 5602798 Live/Registered |
DaySpring Cards, Inc. 2017-09-11 |
 DAYSPRING 78766890 not registered Dead/Abandoned |
Dayspring and Associates 2005-12-05 |
 DAYSPRING 78708181 3112124 Live/Registered |
DaySpring Cards, Inc. 2005-09-07 |
 DAYSPRING 78708157 3112123 Live/Registered |
DaySpring Cards, Inc. 2005-09-07 |
 DAYSPRING 78687844 3112119 Live/Registered |
DaySpring Cards, Inc. 2005-08-08 |
 DAYSPRING 78679817 3112096 Live/Registered |
DaySpring Cards, Inc. 2005-07-27 |
 DAYSPRING 78679791 3112094 Live/Registered |
DaySpring Cards, Inc. 2005-07-27 |
 DAYSPRING 78679766 3112093 Live/Registered |
DaySpring Cards, Inc. 2005-07-27 |
 DAYSPRING 78679727 3112090 Live/Registered |
DaySpring Cards, Inc. 2005-07-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.