J2-100
GUDID 00850020592487
BLUE, NITRILE MEDICAL EXAMINATION GLOVES, LATEX-FREE, POWDER-FREE, ASTM D6319
J2 Medical Supply Inc.
Nitrile examination/treatment glove, non-powdered, non-antimicrobial| Primary Device ID | 00850020592487 |
| NIH Device Record Key | 7ccb1c00-9b2a-48dc-a62c-f560c1160720 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | J2-100 |
| Version Model Number | JVNGWZ4MC |
| Company DUNS | 117257038 |
| Company Name | J2 Medical Supply Inc. |
| Device Count | 100 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com | |
| Phone | 855-615-8633 |
| mariana.pisterman@j2medicalsupply.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00850020592487 [Unit of Use] |
| GS1 | 00850020592524 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K192333
FDA Product Code
| LZA | Polymer Patient Examination Glove |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-06-14 |
| Device Publish Date | 2021-06-04 |
On-Brand Devices [J2-100]
| 00850020592500 | BLUE, NITRILE MEDICAL EXAMINATION GLOVES, LATEX-FREE, POWDER-FREE, ASTM D6319 |
| 00850020592494 | BLUE, NITRILE MEDICAL EXAMINATION GLOVES, LATEX-FREE, POWDER-FREE, ASTM D6319 |
| 00850020592487 | BLUE, NITRILE MEDICAL EXAMINATION GLOVES, LATEX-FREE, POWDER-FREE, ASTM D6319 |
| 00850020592470 | BLUE, NITRILE MEDICAL EXAMINATION GLOVES, LATEX-FREE, POWDER-FREE, ASTM D6319 |
Trademark Results [J2-100]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
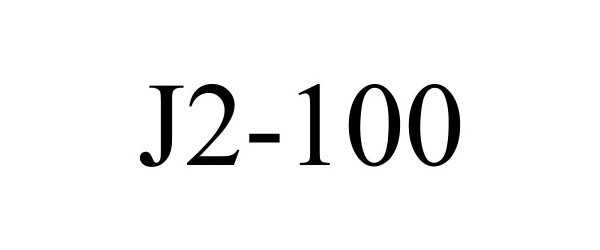 J2-100 90671829 not registered Live/Pending |
J2 Medical Supply Inc. 2021-04-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.