Collagen P.I.N.
GUDID 00850021673017
Microneedling Device
Induction Therapies, LLC
Cosmetic micro-needling electronic handpiece cartridge, professional| Primary Device ID | 00850021673017 |
| NIH Device Record Key | 70f56f6a-9883-4c85-afe4-44ee55e71320 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Collagen P.I.N. |
| Version Model Number | 1 |
| Company DUNS | 098256562 |
| Company Name | Induction Therapies, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00850021673017 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K222199
FDA Product Code
| QAI | Powered Microneedle Device |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-02-13 |
| Device Publish Date | 2023-02-03 |
On-Brand Devices [Collagen P.I.N.]
| 00196852959570 | Microneedling Device |
| 00196852440313 | 1 |
| 00850021673024 | 36 Pin Needle Cartridge |
| 00850021673017 | Microneedling Device |
| 00850021673031 | 24 Pack - 36 Pin Microneedling Cartridge |
| 00850021673048 | 100 Pack - 36 Pin Microneedling Cartridge |
| 00850021673062 | Microneedling Device Kit (Partial) |
| 00850021673055 | Microneedling Device Handpiece (Single) |
| 00850021673079 | Microneedling Device Kit (Ocean) |
Trademark Results [Collagen P.I.N.]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COLLAGEN P.I.N. 90015609 not registered Live/Pending |
Induction Therapies, LLC 2020-06-23 |
 COLLAGEN P.I.N. 88257063 5860909 Live/Registered |
INDUCTION THERAPIES, LLC 2019-01-10 |
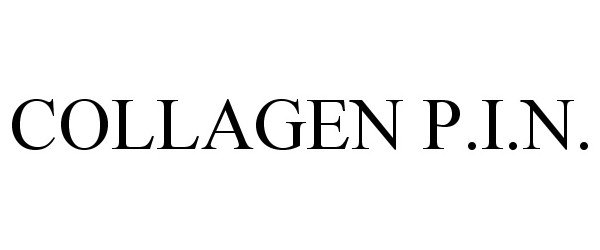 COLLAGEN P.I.N. 87460755 not registered Dead/Abandoned |
Induction Therapies.LLC 2017-05-23 |
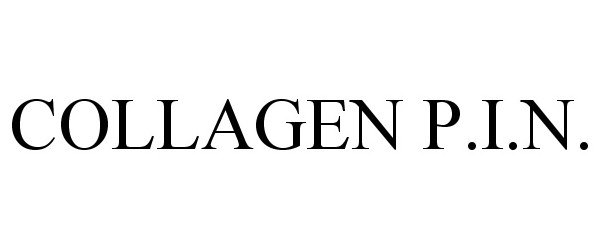 COLLAGEN P.I.N. 86488872 not registered Dead/Abandoned |
Pulse Marketing Communications, LLC 2014-12-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.