Stix
GUDID 00850024942370
Vaginal pH Tests
Get Stix Inc.
Vaginal pH screening IVD, kit, colorimetric dipstick, self-testing| Primary Device ID | 00850024942370 |
| NIH Device Record Key | 5fad0649-2bad-422a-9218-3e7b0276bcba |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Stix |
| Version Model Number | 1 |
| Company DUNS | 117116737 |
| Company Name | Get Stix Inc. |
| Device Count | 2 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co | |
| Phone | +1(999)999-9999 |
| hello@getstix.co |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00850024942325 [Primary] |
| GS1 | 00850024942370 [Unit of Use] |
FDA Product Code
| LNW | Paper, obstetric pH |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-05-24 |
| Device Publish Date | 2021-05-15 |
On-Brand Devices [Stix]
| 00860002060415 | Seven ovulation tests |
| 00860002060408 | Two pregnancy tests |
| 00860002060484 | Three UTI tests |
| 00850024942073 | Three UTI tests |
| 00850024942363 | Pantyliners |
| 00850024942370 | Vaginal pH Tests |
| 00850024942615 | Condom- Single |
Trademark Results [Stix]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 STIX 98180602 not registered Live/Pending |
Stix Golf Co. 2023-09-14 |
 STIX 97976365 not registered Live/Pending |
Siemens Healthcare Diagnostics Inc. 2022-03-25 |
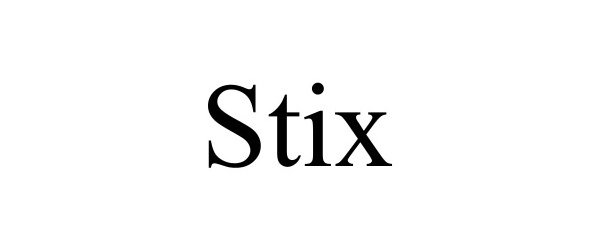 STIX 97678580 not registered Live/Pending |
Downing, Devyn 2022-11-15 |
 STIX 97330575 not registered Live/Pending |
Siemens Healthcare Diagnostics Inc. 2022-03-25 |
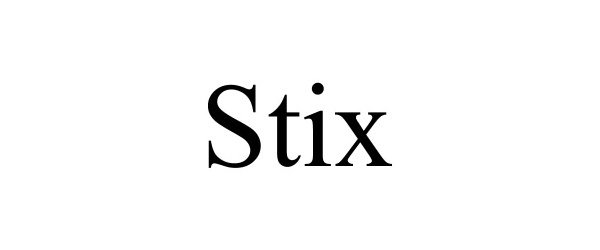 STIX 97313260 not registered Live/Pending |
Media Mynt 2022-03-15 |
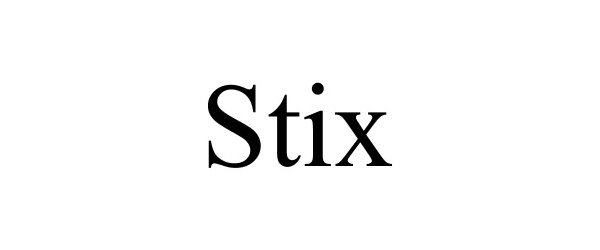 STIX 97063343 not registered Live/Pending |
STIX Enterprises, LLC 2021-10-07 |
 STIX 97055183 not registered Live/Pending |
STIX Gaming Org LLC 2021-09-30 |
 STIX 97016768 not registered Live/Pending |
MANUKA DOCTOR LIMITED 2021-09-08 |
 STIX 90863243 not registered Live/Pending |
EMKSQUARED, LLC 2021-08-03 |
 STIX 90863150 not registered Live/Pending |
EMKSQUARED, LLC 2021-08-03 |
 STIX 90095354 not registered Live/Pending |
Keybob LLC 2020-08-05 |
 STIX 90059116 not registered Live/Pending |
Macro Retail Solutions LLC 2020-07-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.