QOSMIC
GUDID 00850025993883
QOSMIC™ Battery Pack
Enova Illumination, Inc.
Portable/mobile rechargeable battery pack| Primary Device ID | 00850025993883 |
| NIH Device Record Key | 23afeb8c-c1b9-4130-ac61-415cae243769 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | QOSMIC |
| Version Model Number | 5460-800-007 |
| Company DUNS | 080805136 |
| Company Name | Enova Illumination, Inc. |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00850025993883 [Primary] |
FDA Product Code
| HPP | Headlamp, Operating, Battery-Operated |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-08-21 |
| Device Publish Date | 2023-08-11 |
On-Brand Devices [QOSMIC]
| 00850025993937 | Single Battery Charger |
| 00850025993920 | QOSMIC™ SR Replacement Pad Set |
| 00850025993913 | QOSMIC™ DR Replacement Pad Set |
| 00850025993906 | QOSMIC™ DRO Replacement Pad Set |
| 00850025993890 | QOSMIC™ Twist Cable |
| 00850025993883 | QOSMIC™ Battery Pack |
| 00850025993876 | QOSMIC™ LED Headlight Only SR |
| 00850025993869 | QOSMIC™ LED Headlight Only DR |
| 00850025993852 | QOSMIC™ LED Headlight Only DRO |
| 00850025993951 | QOSMIC™ SRO Replacement Pad Set |
| 00850025993944 | QOSMIC™ LED Headlight Only SRO |
Trademark Results [QOSMIC]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
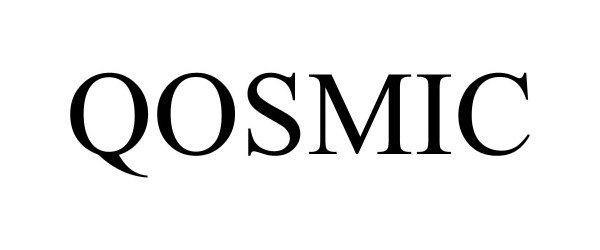 QOSMIC 98090089 not registered Live/Pending |
Stryker Corporation 2023-07-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.