Steri-Shield
GUDID 00850040097153
Wingers-A, SM Case
Steri-Shield Products
Dental x-ray film holder, single-use| Primary Device ID | 00850040097153 |
| NIH Device Record Key | 78fc0b7e-8d74-420a-8219-0e442ec6d1bf |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Steri-Shield |
| Version Model Number | 088-028 |
| Company DUNS | 026709913 |
| Company Name | Steri-Shield Products |
| Device Count | 24 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00850040097153 [Unit of Use] |
| GS1 | 10850040097150 [Primary] |
FDA Product Code
| EGZ | Holder, Film, X-Ray |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-06-13 |
| Device Publish Date | 2022-06-03 |
On-Brand Devices [Steri-Shield]
| 00850040097283 | Wingers-H, Sm (Use with Aimer Ring) Case |
| 00850040097276 | Aimer Ring & Bar Set - Case |
| 00850040097269 | Smoothies - Case |
| 00850040097252 | Wingers I.B. (Integrated Barrier) - Case |
| 00850040097245 | Wing-A-Ray - Case |
| 00850040097238 | Anterior Bar - Case |
| 00850040097221 | Posterior Bar - Case |
| 00850040097214 | Aimer Ring - Case |
| 00850040097207 | Wingers-V - SM, Case |
| 00850040097191 | Wingers-V - LG, Case |
| 00850040097184 | Wingers-HS, SUNI - LG, Case |
| 00850040097177 | Wingers-HS, SUNI - SM, Case |
| 00850040097160 | Wingers-H, LG (Use with Aimer Ring) Case |
| 00850040097153 | Wingers-A, SM Case |
| 00850040097146 | Wingers-A, LG Case |
| 00850040097139 | Wingers-P, SM, Case |
| 00850040097122 | Wingers-P, LG Case |
| 00850040097115 | Wingers-H "Classic" - SM, Case |
| 00850040097108 | Wingers-H "Classic" LG -Case |
| 00850040097092 | R.S. Barrier, Nitrile, Case |
| 00850040097085 | T-Bar Barrier, Nitrile, Case |
| 00850040097078 | C-Bar Barrier, Nitrile, Case |
| 00850040097061 | Curelastic (med/lg), Non-latex, Case |
| 00850040097054 | Curelastic (sm/med), Non-latex, Case |
| 00850040097030 | R.S. Barrier, Latex, Case |
| 00850040097023 | Multi-Use Barrier, Latex, Case |
| 00850040097016 | C-Bar Barrier, Latex, Case |
| 00850040097009 | T-Bar Barrier, Latex, Case |
Trademark Results [Steri-Shield]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
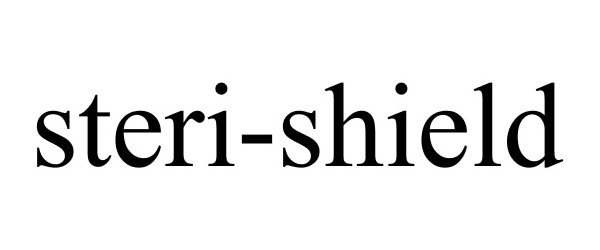 STERI-SHIELD 88090848 5715091 Live/Registered |
Steri-Shield Inc. 2018-08-23 |
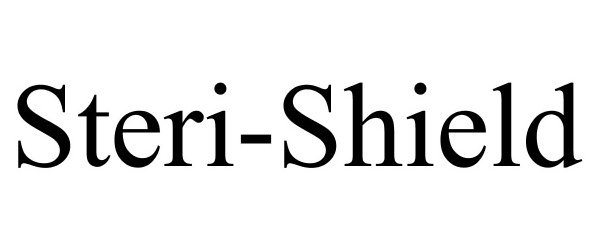 STERI-SHIELD 77027919 3356891 Dead/Cancelled |
Portola Packaging, Inc. 2006-10-24 |
 STERI-SHIELD 74568273 1950561 Live/Registered |
Dentex Ltd. Partnership 1994-08-31 |
 STERI-SHIELD 74057722 1777108 Live/Registered |
BIO-MEDICAL DEVICES, INC. 1990-05-10 |
 STERI-SHIELD 73799288 1638981 Dead/Cancelled |
COOK (CANADA) INC. 1989-05-11 |
 STERI-SHIELD 73572963 1402121 Dead/Cancelled |
HENSLEY AND SWANSON PARTNERS 1985-12-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.