Poly-Med
GUDID 00850054694911
POLYCONVERSIONS, INC.
Surgical/examination garment kit| Primary Device ID | 00850054694911 |
| NIH Device Record Key | 5925966f-7f17-486c-99ed-6fbd9b3a2b2b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Poly-Med |
| Version Model Number | PLG-4250 |
| Company DUNS | 825056401 |
| Company Name | POLYCONVERSIONS, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00850054694911 [Primary] |
FDA Product Code
| OEA | Non-Surgical Isolation Gown |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-09-27 |
| Device Publish Date | 2023-09-19 |
Devices Manufactured by POLYCONVERSIONS, INC.
| 00850054694911 - Poly-Med | 2023-09-27 |
| 00850054694911 - Poly-Med | 2023-09-27 |
| 00860006079185 - VR | 2021-07-22 VR Thumb Loop Gown, Large 4 Mil Green |
| 00860006079192 - VR | 2021-07-22 VR Thumb Loop Gown, Large 4 Mil Green, Case - 50 Ct. |
| 00860006079178 - VR | 2021-07-09 VR Thumb Loop Gown, Regular 4 Mil Green |
| 10860006079175 - VR | 2021-07-09 VR Thumb Loop Gown, Regular 4 Mil Green, Case - 50 Ct. |
| 00860006079109 - VR | 2020-12-29 Sleeve Gloves 15 Mil Extra Large Green Unlined |
| 00860006079116 - VR | 2020-12-29 Sleeve Gloves 15 Mil Large Green Unlined |
| 00860006079123 - VR | 2020-12-29 Sleeve Gloves 15 Mil Medium Green Unlined |
Trademark Results [Poly-Med]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
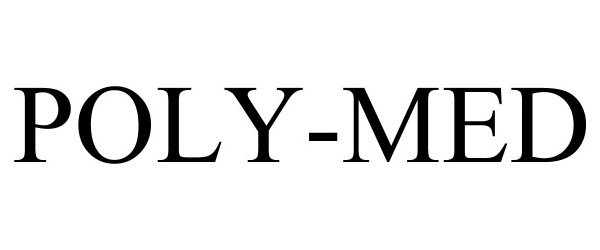 POLY-MED 97212476 not registered Live/Pending |
Poly-Med, Inc. 2022-01-11 |
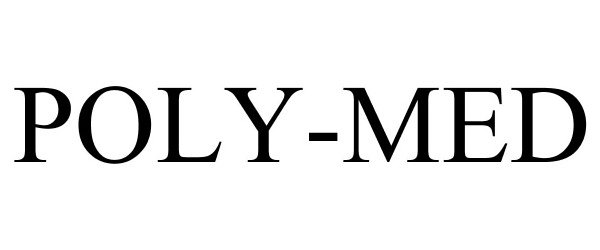 POLY-MED 85206607 4086556 Live/Registered |
Poly-Med, Inc. 2010-12-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.