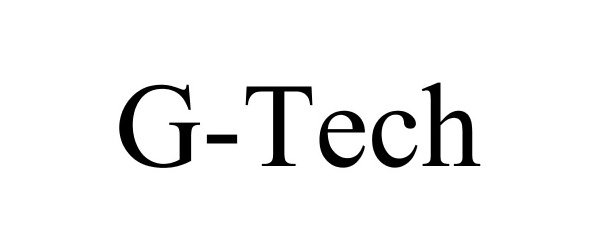G-Tech
GUDID 00850057194043
Electrology Hair Removal (Needle Epilation)
Clareblend, Inc.
Professional hair-removal electrolysis system| Primary Device ID | 00850057194043 |
| NIH Device Record Key | d9a4a215-f058-467e-8b17-8311b6889d8e |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | G-Tech |
| Version Model Number | 2021-GT |
| Company DUNS | 139701114 |
| Company Name | Clareblend, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | true |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Dry |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00850057194043 [Primary] |
FDA Product Code
| KCW | EPILATOR, HIGH FREQUENCY, NEEDLE-TYPE |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-12-22 |
| Device Publish Date | 2023-12-14 |
Devices Manufactured by Clareblend, Inc.
| 00850057194005 - Elegance+ | 2023-12-22 Electrology Hair Removal (Needle Epilation) |
| 00850057194036 - Elegance | 2023-12-22 Electrology Hair Removal (Needle Epilation) |
| 00850057194043 - G-Tech | 2023-12-22Electrology Hair Removal (Needle Epilation) |
| 00850057194043 - G-Tech | 2023-12-22 Electrology Hair Removal (Needle Epilation) |
| 00850057194050 - Essence | 2023-12-22 Electrology Hair Removal (Needle Epilation) |
| 00850057194067 - EZ3 | 2023-12-22 Electrology Hair Removal (Needle Epilation) |
| 00850057194074 - Lamprobe | 2023-12-22 Electrology Hair Removal (Needle Epilation) |
| 00850057194081 - Skin Classic | 2023-12-22 Electrology Hair Removal (Needle Epilation) |
| 00850057194098 - Thermoclear | 2023-12-22 Electrology Hair Removal (Needle Epilation) |
Trademark Results [G-Tech]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.