Neoscope
GUDID 00851545007524
NEOFLEX-FLEXIBLE VIDEO HYSTEROSCOPE
PROSURG INC
Flexible video hysteroscope| Primary Device ID | 00851545007524 |
| NIH Device Record Key | 8ef338ae-91e0-4cc2-b483-90f3ce99c5d8 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Neoscope |
| Version Model Number | NFH 17 |
| Company DUNS | 188684609 |
| Company Name | PROSURG INC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 408 945 4044 |
| prosurg_qa@sbcglobal.net |
Device Dimensions
| Catheter Gauge | 17 French |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00851545007524 [Primary] |
| GS1 | 10851545007521 [Package] Package: Box [1 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K962593
FDA Product Code
| FAS | Electrode, Electrosurgical, Active, Urological |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2020-04-23 |
| Device Publish Date | 2016-09-23 |
On-Brand Devices [Neoscope]
| 00851545007623 | NEOFLEX-FLEXIBLE VIDEO URETEROSCOPE |
| 00851545007487 | NEO- HYSTEROSCOPE OPERATING SHEATH |
| 00851545007470 | NEO-CYSTOSCOPE , DIAGNOSTIC SHEATH |
| 00851545007463 | NEO-CYSTOSCOPE , DIGITAL VIDEOSCOPE |
| 00851545007579 | NEOFLEX - FLEXIBLE VIDEO BRONCHOSCOPE |
| 00851545007562 | NEOFLEX- FLEXIBLE VIDEO BRONCHOSCOPE |
| 00851545007531 | NEO -CYSTOSCOPE, OPERATING SHEATH |
| 00851545007524 | NEOFLEX-FLEXIBLE VIDEO HYSTEROSCOPE |
| 00851545007494 | NEO- HYSTEROSCOPE, DIGITAL VIDEOSCOPE |
| 00851545007548 | NEOFLEX -FLEXIBLE VIDEO CYSTOSCOPE |
| 00851545007500 | NEO-HYSTEROSCOPE DIAGNOSTIC SHEATH |
Trademark Results [Neoscope]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
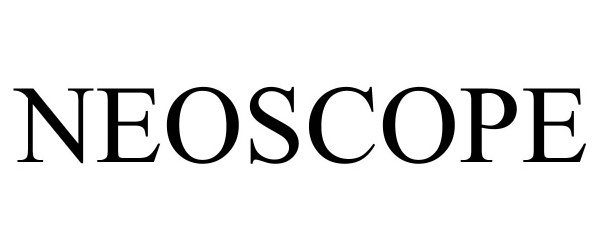 NEOSCOPE 90510116 not registered Live/Pending |
Schlumberger Technology Corporation 2021-02-04 |
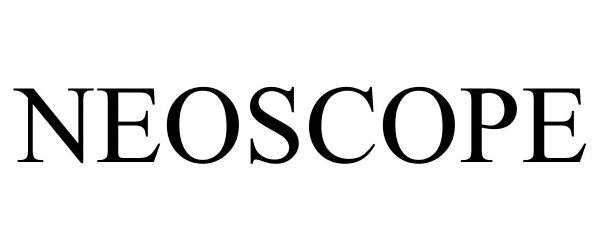 NEOSCOPE 85582333 4679245 Live/Registered |
Schlumberger Technology Corporation 2012-03-28 |
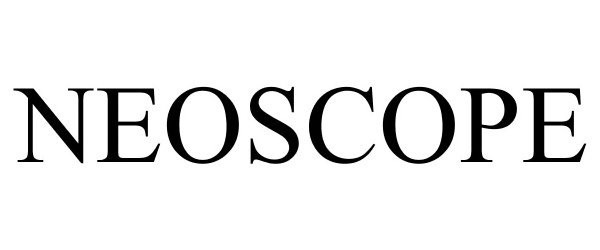 NEOSCOPE 85010694 not registered Dead/Abandoned |
Elliott, Stephen J. 2010-04-09 |
 NEOSCOPE 78085125 not registered Dead/Abandoned |
Magna Fortis Corporation 2001-09-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.