ONDAMED 001-55-0101
GUDID 00852318007550
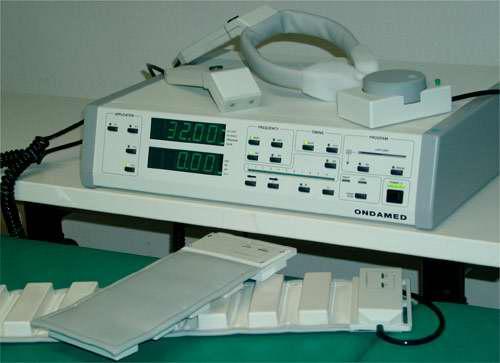
ONDAMED Biofeedback System with MA4, MA8 and Flex
ONDAMED INC.
Biofeedback system| Primary Device ID | 00852318007550 |
| NIH Device Record Key | a4ffa1ea-05ef-4309-a5a2-881c99f1e79c |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | ONDAMED |
| Version Model Number | 001-55-0101 |
| Catalog Number | 001-55-0101 |
| Company DUNS | 841720167 |
| Company Name | ONDAMED INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | MR Unsafe |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 8455340456 |
| support@ondamed.net |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00852318007550 [Primary] |
FDA Product Code
| HCC | Device, Biofeedback |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2018-03-29 |
| Device Publish Date | 2016-09-24 |
On-Brand Devices [ONDAMED]
| 00852318007574 | ONDAMED Biofeedback System Basic |
| 00852318007567 | ONDAMED Biofeedback System with Flex |
| 00852318007550 | ONDAMED Biofeedback System with MA4, MA8 and Flex |
| 00852318007543 | ONDAMED Biofeedback System with MA8 |
| 00852318007536 | ONDAMED Biofeedback System with MA4 |
| 00852318007529 | ONDAMED Biofeedback System with MA4 and Flex |
| 00852318007512 | ONDAMED Biofeedback System with MA8 and Flex |
| 00852318007505 | ONDAMED Biofeedback System with MA4 and MA8 |
| 00852318007215 | ONDAMED Flex Applicator |
| 00852318007055 | ONDAMED Regulator |
| 00852318007048 | ONDAMED MA8 Applicator |
| 00852318007031 | ONDAMED MA4 Applicator |
| 00852318007024 | ONDAMED Neck Applicator |
| 00852318007017 | ONDAMED Hand Applicator |
| 00852318007000 | ONDAMED Main Unit |
Trademark Results [ONDAMED]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ONDAMED 85189348 not registered Dead/Abandoned |
Ondal Holding GmbH 2010-12-02 |
 ONDAMED 78215135 2883198 Live/Registered |
Ondamed, Inc. 2003-02-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.